The birth of the Periodic Table took place at the Chemiker Kongress (Chemical Congress) of September 3-5, 1860 in Karlsruhe, Germany.
Since Lavoisier’s time, scientists had been searching for ways to explain the chemical and physical similarities in elements, which mysteriously repeated at regular intervals with increasing atomic weight. Schematics included everything from a 45-degree spiral – the telluric helix – to the law of “Law of Octaves”. Part of the problem was that the atomic weight was often calculated as a multiple (or fraction) of the apparent equivalent weight of an element. Even the formula for water – H2O, 2 hydrogen atoms to 1 oxygen atom – was up for debate.
It was at this meeting, the Italian chemist Stanislao Cannizzaro (1826-1910) reintroduced a theory proposed by Amedeo Avogadro (1776-1856) in which valence could be used to establish a self-consistent set of true, absolute atomic weights. Two chemists in the audience, the German chemist Lothar Meyer (1830-1895) and the Russian chemist Dimitri Mendeleev (1834-1907), were quick to recognize how these corrected numbers allowed for a new ordering of the elements.
Each subsequently proposed Periodic Tables of their own, but it was Mendeleev’s version, published a year before Meyer’s, that eventually became standard. Mendeleev had been so bold as to predict not only the existence of new elements — including scandium, gallium, and germanium — but also the properties of each. The Periodic Table as we know it today came to its final form with the introduction of atomic numbers in 1912.
Explore the elements by decade:
- 1865-1874: helium
- 1875-1884: gallium, holmium, ytterbium, scandium, samarium, gadolinium, thulium
- 1885-1894: praseodymium, neodymium, germanium, dysprosium, argon, fluorine
- 1895-1904: europium, neon, krypton, xenon, polonium, radium, radon, actinium
- 1905-1914: lutetium
- 1915-1924: hafnium, protactinium
- 1925-1934: rhenium
Read more: "The Creation of the Periodic Table," by James Marshall, a Chem 13 News article (November 2019).
1865-1874: helium
Helium, 2

North York, Ontario, Canada
Teacher: Alicja Koprianiuk
Artist: Jakub Brol
My depiction of helium is the following: on the right side of the hexagon there is a hot air balloon which shows the French flag. I chose to do the French flag for the balloon because helium was discovered by a French astronomer, Pierre Jules César Janssen. On the other side of the hexagon I drew the Sun because it was where helium was first found. More specifically it was discovered as a yellow line on the border of the Sun. Additionally, I added a magenta glow as the flames representing the colour that helium emits in a discharge tube.
Note: There has been a correction to the original poster. Thulium (Tm, 69) was shifted from 1865-1874 to 1875-1884. The website, interactive pdf and downloadable poster are all updated to reflect this change.
1875-1884: gallium, holmium, ytterbium, scandium, samarium, gadolinium, thulium
Gallium, 31

Hudsonville, Michigan, U.S.A.
Teacher: Doug Ragan
Artist: Sophia Putman
Gallium was discovered in 1875 by Paul - Émile LeCoq de Boisbaudran by means of using a spectroscope which shines white light through a prism, breaking up the light into its individual color wavelengths through refraction. My display includes separated rainbow colors to represent how he discovered the element. Gallium's (Ga) atomic number is 31. Gallium is a metal that is a brittle solid at low temperatures and liquid at temperatures above 85.57 ⁰F (29.76 ⁰C). I included a dripping liquid cutout to represent the elements liquid state, and crunched solid cutouts to show it as a solid.
Holmium, 67

Geraldton, Western Australia, Australia
Teacher: Mrs. Elisabete Costa
Artist: Ashleigh Naisbitt and Emily MacPherson
Our design depicts the discovery and magnetic properties of holmium. The three men who discovered the element are illustrated with holmium's spectroscopic colour lines. The most predominant figure is Swedish Per Teodor Cleve the main discoverer of the element. Holmium atoms are represented through multicoloured circles of white and yellow dots. The magnet and surrounding magnetic fields illustrate the element’s magnetic properties. The line spectrum at the base of the artwork represent the absorption lines of holmium. The wavelengths of the absorption lines allowed the discovery of holmium. Blue and yellow undertones are symbolic of Sweden's flag.
Ytterbium, 70
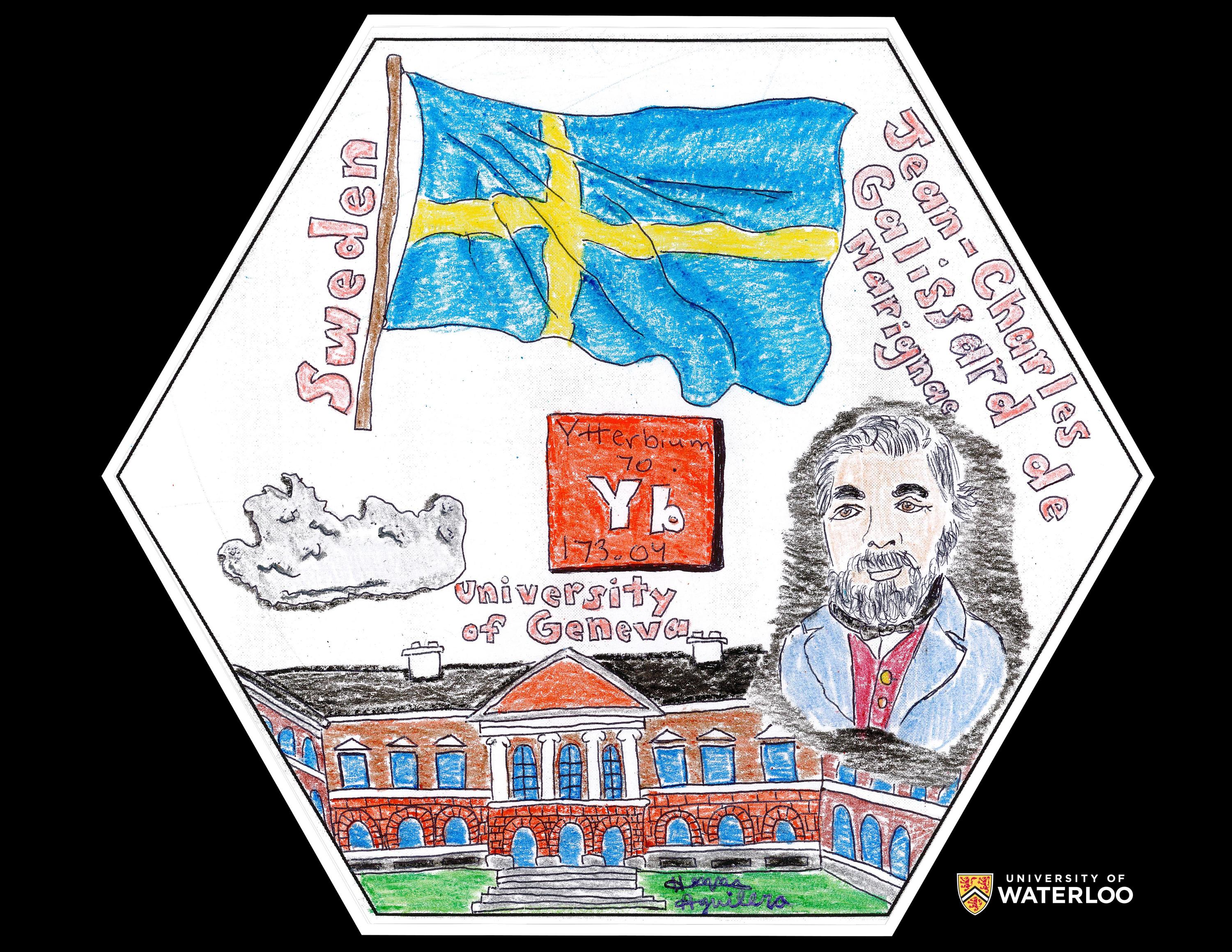
Jamestown, North Carolina, U.S.A.
Teacher: Elizabeth Sunshine
Artist: Henna Aguilera
When creating my artwork, I researched the element of ytterbium, its history, and discovery. Ytterbium was discovered by Jean Charles Galissard de Marignac and I decided he deserved credit for this contribution to science, so I drew him on the right side. Since ytterbium’s discovery was in a Swedish town, I added the Swedish flag. The experiments which discovered ytterbium, occurred at the University of Geneva, so I drew the university profile at the bottom. To the right, I drew how ytterbium appears in nature, and I finalized everything by adding the symbol representation in the middle of the drawing.
Scandium, 21
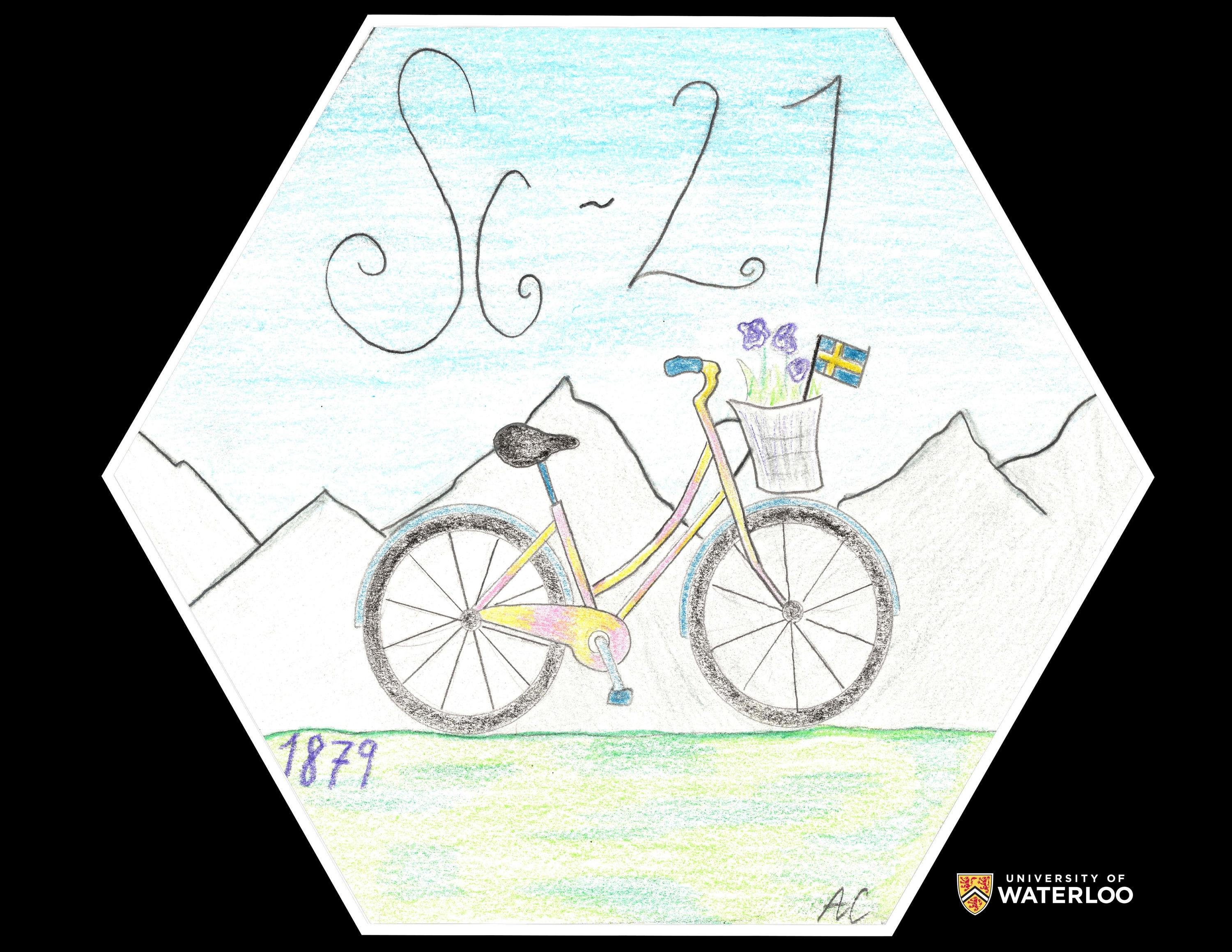
Pittsburgh, Pennsylvania, U.S.A.
Teacher: Brittney Livesay
Artist: Ava Culley
Scandium is the twenty-first element on the periodic table. It was discovered in 1879 by Lars F. Nilson. The element received the name ‘Scandium’ because it was found in Sweden which is part of the Scandinavian Region. The reason there is a mountain range on the tile is because there are mountains in the Scandinavian Region. The pink and blue bike represents a common hobby of Swedish citizens. Scandium is also used in bicycles, along with baseball bats. The Swedish Flag in the bike basket shows the country where Scandium was discovered.
Samarium, 62
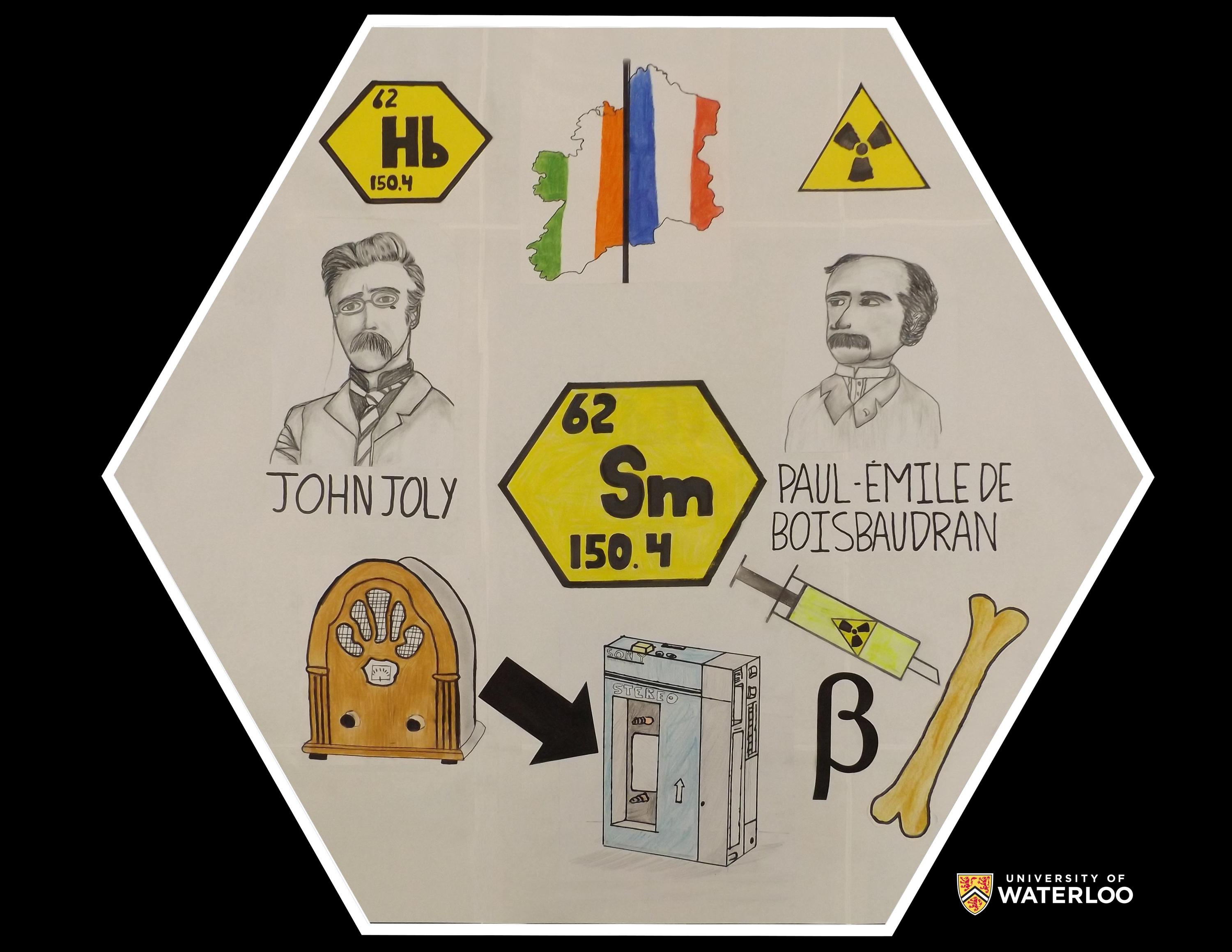
Swords, Co Dublin, Republic of Ireland
Teacher: Seán Kelleher
Artist: Transition Year 1 Science Students
John Joly was an Irish geologist, and physicist, who “discovered” the element Hibernium while studying a piece of granite in 1907. (Samarium had previously been discovered in 1853). Joly was a pioneer in radioactive dating of the Earth, and co-developed the “Dublin Method” of intravenous radiotherapy: Samarium is a beta emitter used in bone cancer radiotherapy. Miniaturization of magnets in speakers used in the transition from free standing radios, to Walkmans and other portable audio devices, involved the addition of samarium.
Gadolinium, 64

Istanbul, Tuzla, Turkey
Teacher: Gulsen Sokullu
Artist: Alara Aydin
I started off the drawing with a television because gadolinium is used in coloured television. I first wanted to draw veins (since gadolinium compounds are used in MRI) but I figured that would be a little complicated. The colors of the symbol and number are from a picture of gadolinium. Last but not least, the discoverer of gadolinium (Jean Charles Galissard de Marignac) must definitely have a place in the artwork.
Note: There has been a correction to the original poster. Thorium (Th, 90) was moved from the decade 1875-1884 to 1825-1834. The website, interactive pdf and downloadable poster are all updated to reflect this change.
Thulium, 69
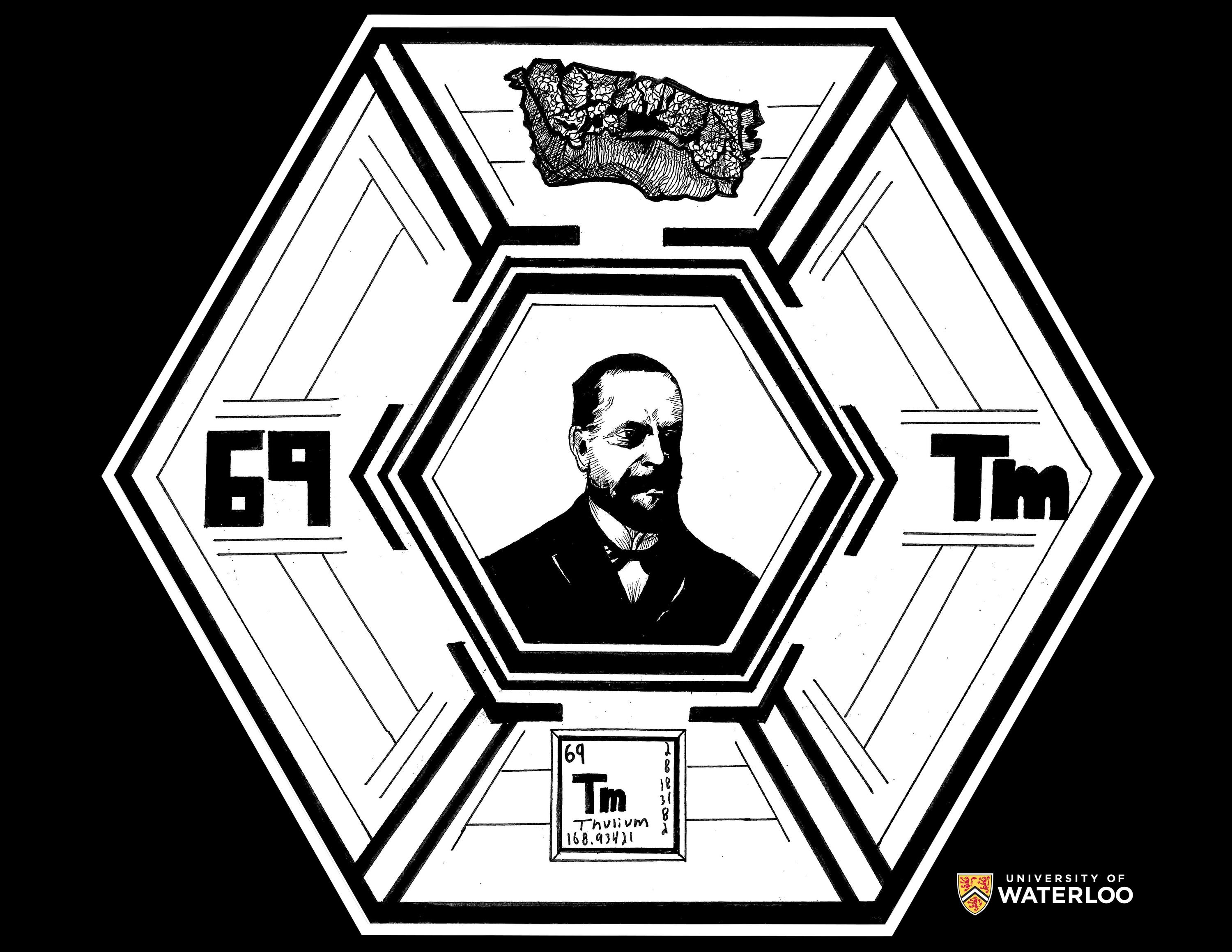
Tampa, Florida, U.S.A.
Teacher: Allison Mercer
Artist: Harrison Alster
Per Teodor Cleve discovered thulium and is featured in the center of the artwork. At the top is a drawing of thulium and surrounding Per Teodor Cleve are the atomic number 69, the symbol Tm, and the classic periodic table tile of thulium. Pen and ink.
1885-1894: praseodymium, neodymium, germanium, dysprosium, argon, fluorine
Praseodymium, 59
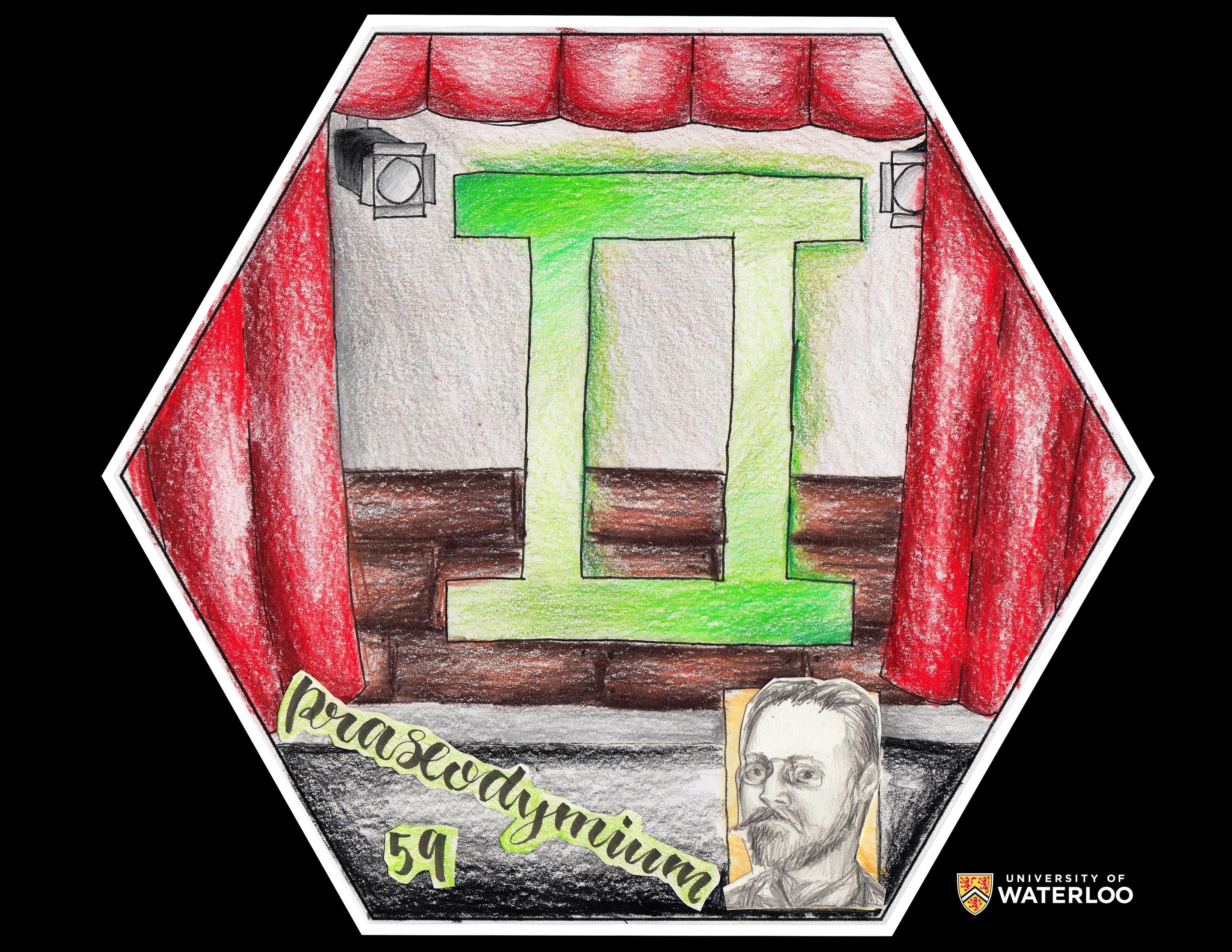
Pflugerville, Texas, U.S.A.
Teacher: Christina Hotchkin
Artists: Anastasia T., Kayla K. and Brenda O.
We found out that our element is used in stage lighting, the light bulb part of it, and was discovered by Carl Auer von Welsbach. The Greek translation is the “green twin” due to the green oxide it produces. We decided to make the background a stage with the types of stage lights that have our element with it and on the stage we have the symbol for the green twins (Roman numeral for two colored green). We colored the tile in a way that it looks like it is glowing due to the stage lighting.
Neodymium, 60

Seoul, South Korea, South Korea
Teacher: Kim Stuart
Artist: Irene Cho
Neodymium is used in objects that we use in our daily lives, such as lighter flints and magnets. The weirdly shaped green on the far right was made to resemble the northern part of Austria, where neodymium was discovered in 1885 by Carl Auer von Welsbach. In the center, there is a large drawing of the metal neodymium in a glass vial. I tried to make it more like the actual thing by using a metallic silver-coloured marker and adding pencil marks (the silver colour did not show up in the scanned copy.)
Germanium, 32
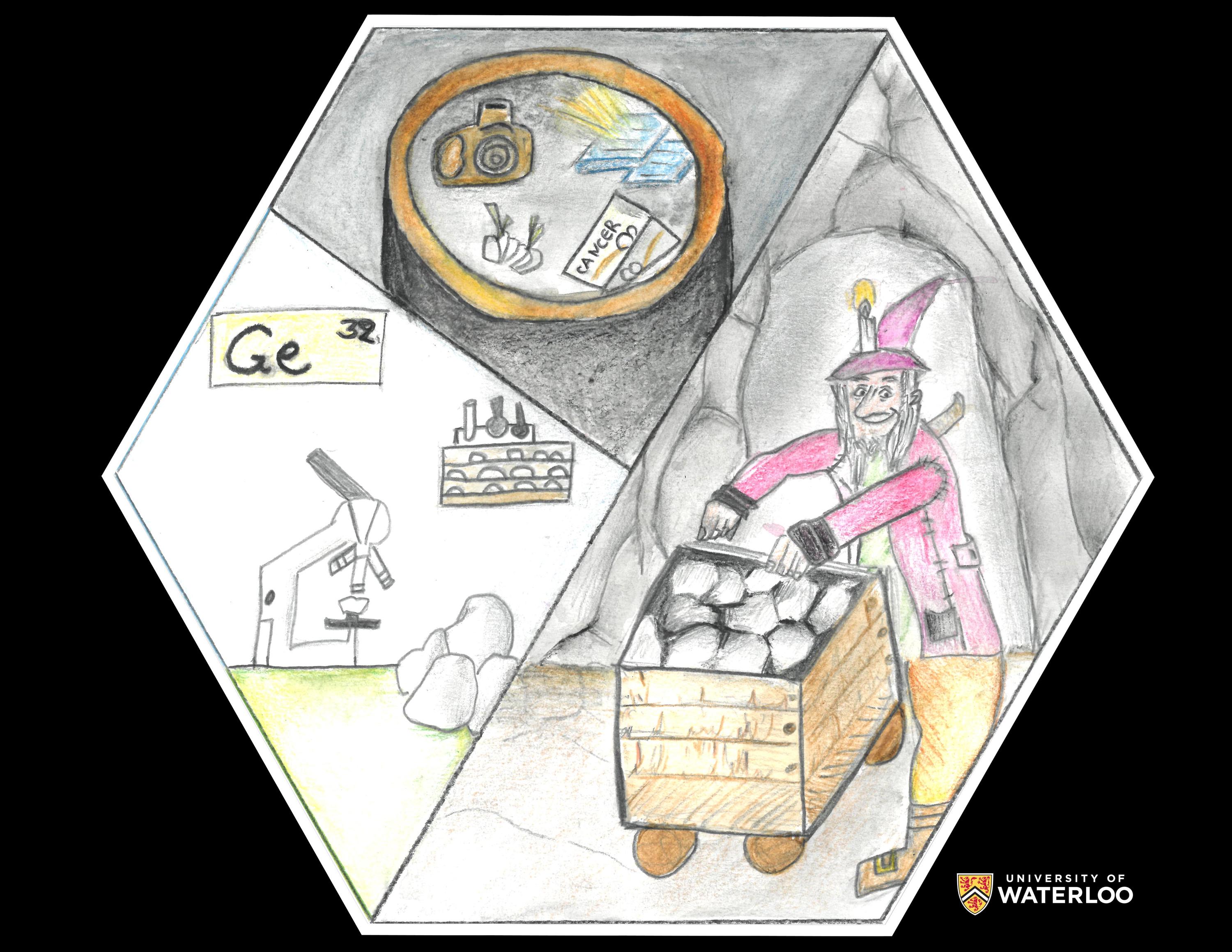
Mytilene, Lesvos, Greece
Teachers: Vasiliki Thomaidi and Maria Giannikou
Artist: Anna - Maria Tsokarou
This drawing was made in the context of chemistry and art lessons. When I heard about the topic, the first thing that came into my mind was a board game I used to play with my friends. It was about a few miners who were trying to find gold, just like Clemens Winkler who discovered germanium in a mine in 1886. So, the first part of my drawing depicts a miner. Next, I decided to draw some other applications of germanium. Generally, my drawing develops chronologically the whole story of Germanium.
Dysprosium, 66

Lexington, South Carolina, U.S.A.
Teacher: Sam Oxley
Artist: Elizabeth Mulligan
The dysprosium artwork features a large Eiffel Tower on the right because the chemist that discovered this element, Paul Emile Lecoq de Boisbaudran, was French. On one leg of the tower, the year dysprosium was discovered (1886) is written. The atomic number is hanging from the top of the tile. Dysprosium is highly magnetic, so the symbol is shown being drawn towards a magnet. Along the bottom, there is a drawing of the element’s natural gray appearance. Red lasers and wind turbines are illustrated to emphasize the element’s practical applications.
Argon, 18

Arnprior, Ontario, Canada
Teacher: Cheryl Welbanks
Artist: Kaelyn Proulx
The name argon comes from a Greek word meaning lazy or inert because the element did not react very easily. We represented the laziness of the element with the image of a sloth. The crown represents that the element is a noble gas. The lightbulb represents how argon is used in incandescent lightbulbs so the filaments won't burn out. The background colour was chosen as it represents the range of colours that argon appears in an electric field. Finally, the bottom image is a canister of gas as argon is used as a shielding gas in welding.
Fluorine, 9

San Antonio, Texas, United States of America
Teacher: Dr. G. Robert Shelton
Artist: Kiarah Craft
Fluorine was first discovered by a French chemist named Henri Moissan in 1886. He isolated and discovered this element by passing electricity through hydrofluoric acid and potassium fluoride using electrolysis.
1895-1904: europium, neon, krypton, xenon, polonium, radium, radon, actinium
Europium, 63
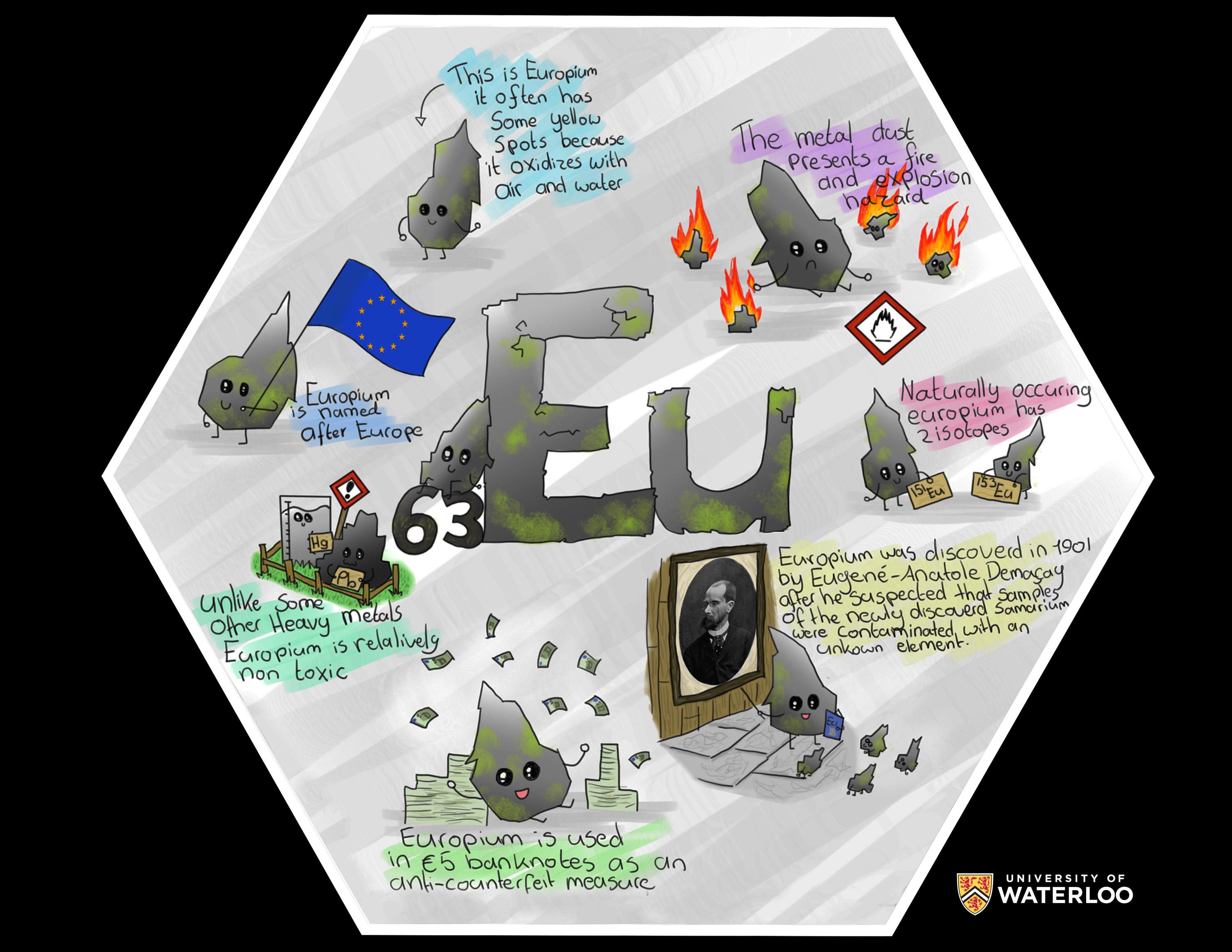
Hoogezand, Groningen, Netherlands
Teacher: Dr. Michel Wijnhold
Artist: Caitlyn Vijlbrief
Europium is one of the more reactive elements from the Lanthanide series. It was discovered through early spectroscopy experiments in 1890 by P.E. Lecocq de Boisbaudran, but its discovery is credited to Eugène-Anatole Demarçay who was the first to isolate the element in its pure form in 1896.
When the countries of the European Union introduced their common currency (the euro), the element named after Europe was chosen to secure the 5 euro banknotes because Eu-compounds emit fluorescent light. This secret was revealed by Dutch chemists as they played around in the lab with the new currency. They never identified the exact compound because that would involve destroying a bank note, which constitutes a crime.
In my class, I have a very quiet student, who was always drawing and sketching things. Therefore I asked her to make this tile, since it would combine her love for art with her chemistry classes.
Neon, 10
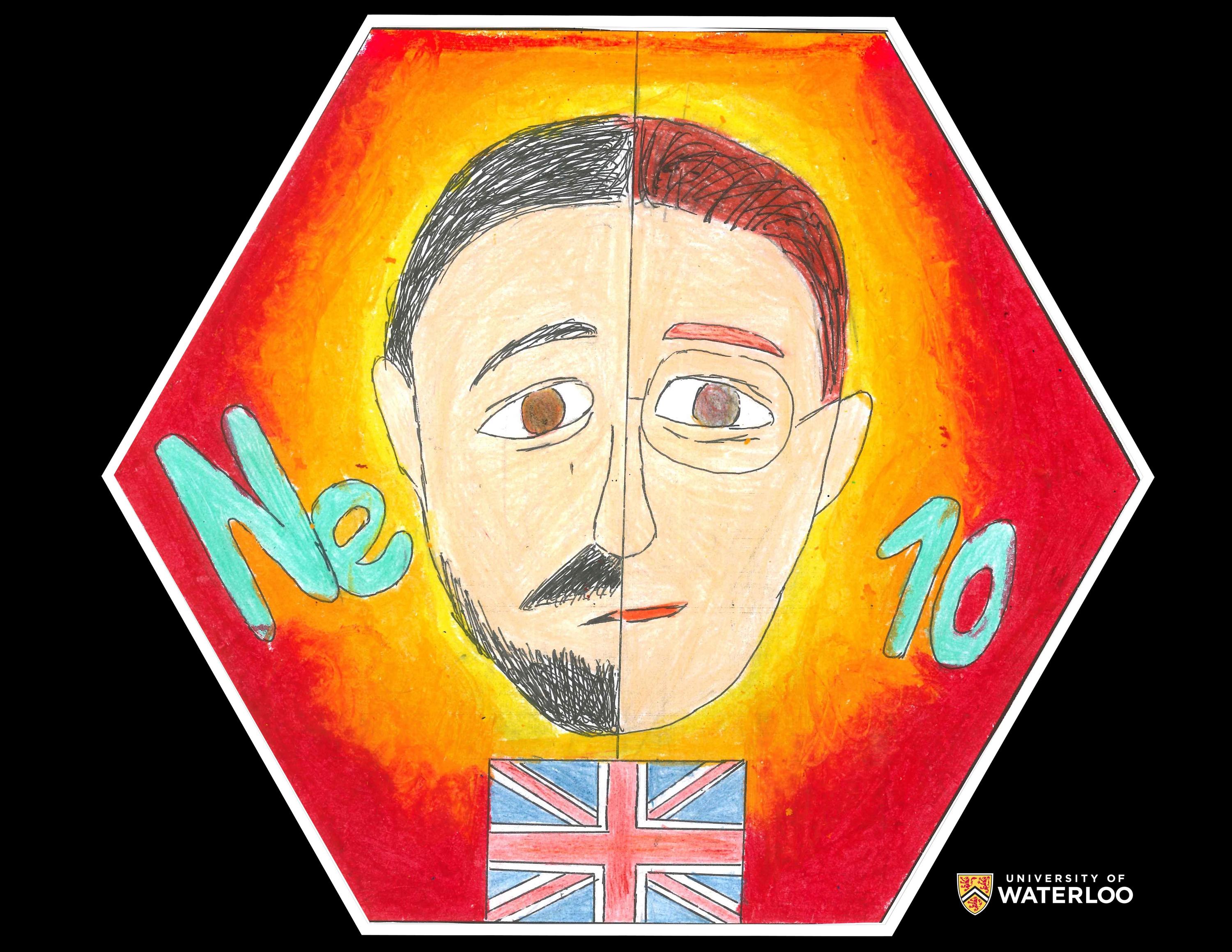
Ho Chi Minh City, Vietnam
Teacher: Alex Love
Artist: Nguyen Truong Thu Ngoc
English scientists Sir William Ramsay and Morris Travers, the two faces in the centre of the tile, froze a sample of argon using liquid air in 1898. They collected the first amount of gas that had evaporated, put the sample into an atomic spectrometer and observed a red light glowing from the gas. This bright colour is the background presented in the artwork. Moreover, neon is the fifth most abundant element in the universe but it is rare on Earth. This element is used to make colourful and dashing neon lights, which can be seen in commercial signs.
Krypton, 36
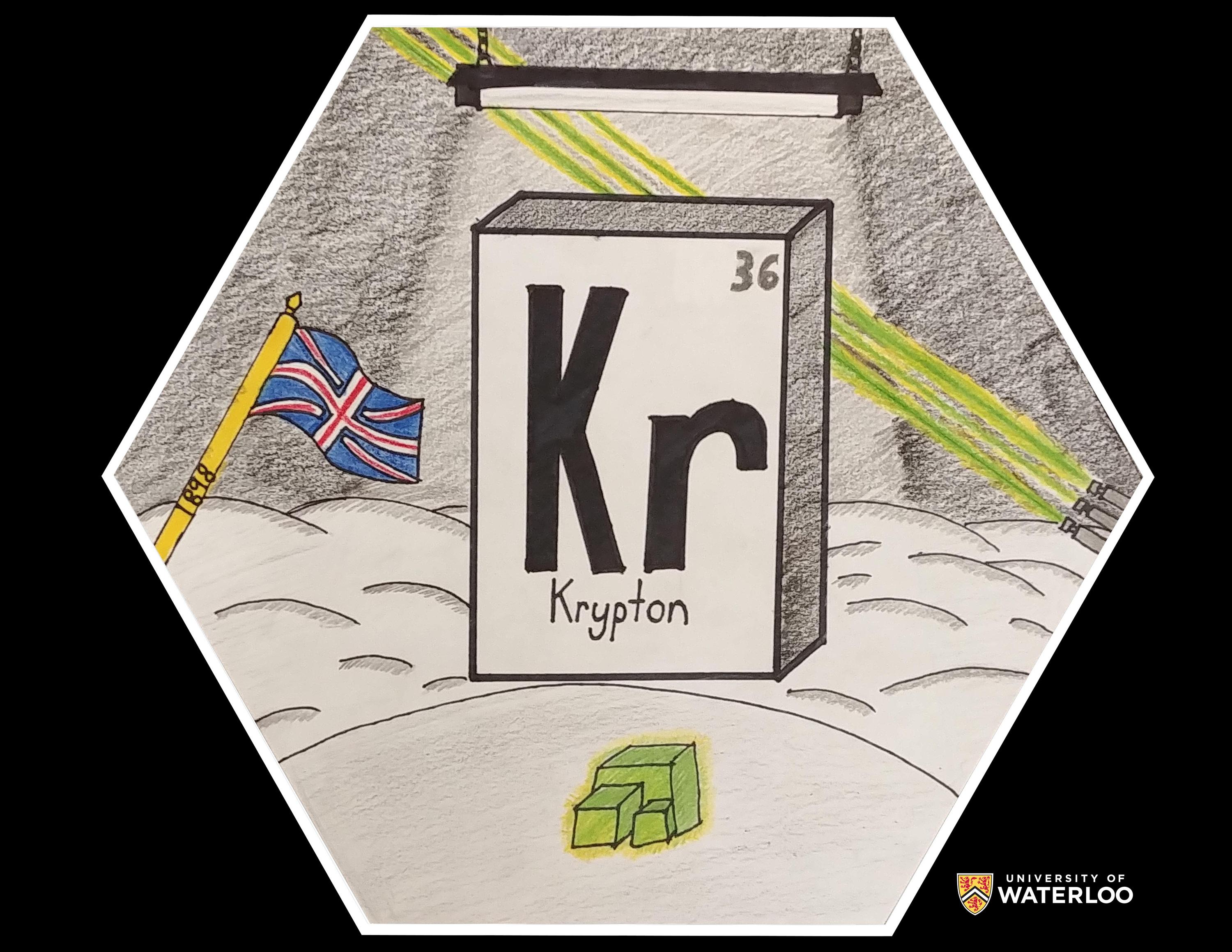
Enderby, British Columbia, Canada
Teacher: Mrs. Annette Toop
Artist: Tristan Baumle
My initial idea was to have the element's symbol in the middle like a regular periodic table. Through my research I decided to include as many different uses of krypton I could. I started with the fluorescent light on the top of the artwork. In the background I included lasers, another use of krypton. The green cubic crystal shows krypton in its crystallized form. Finally, I put in the UK flag and the year for its discovery, 1898.
Xenon, 54
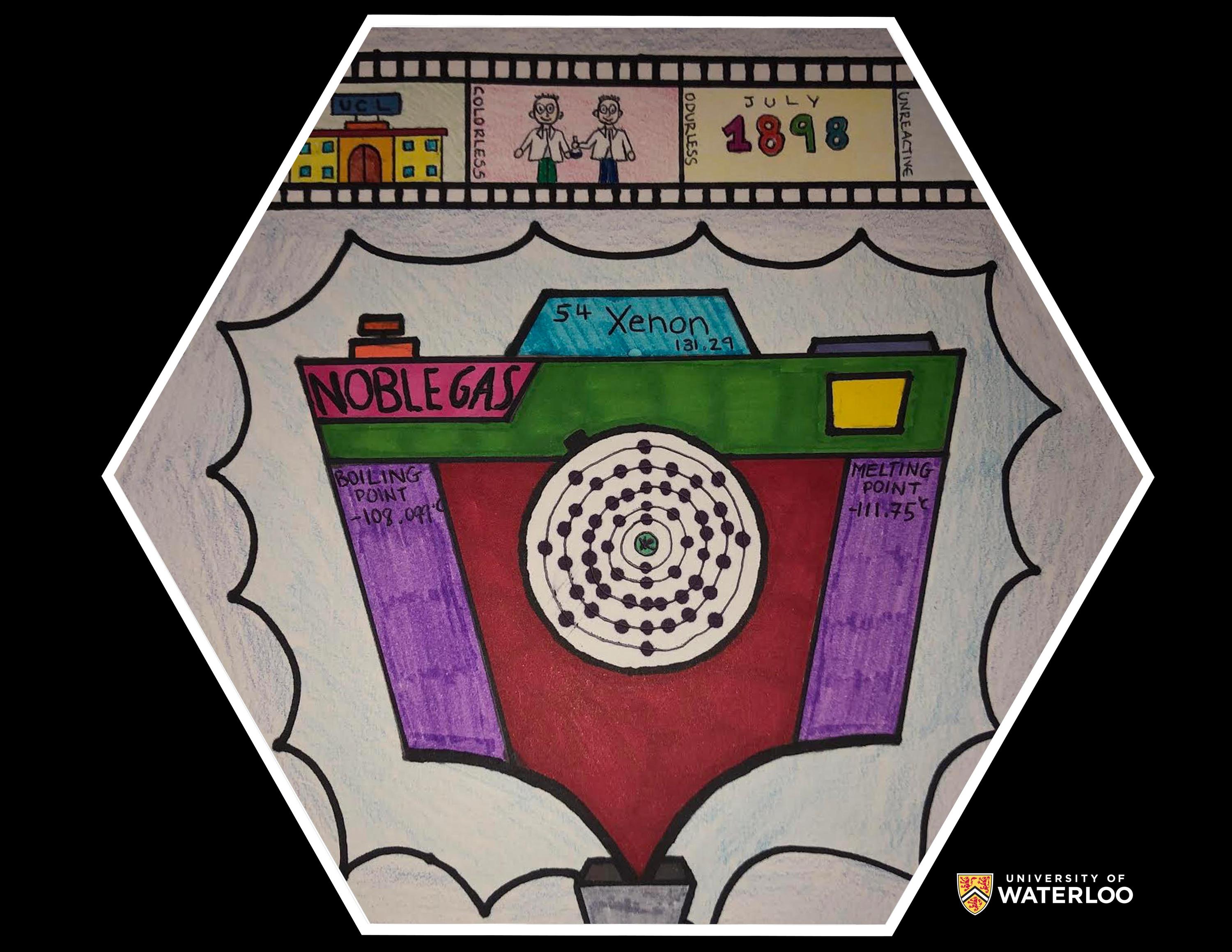
Oberursel, Hessen, Germany
Teacher: Mr. Vee Kummari
Artist: Bronwyn Sandfield
At the top of my tile I drew a photo film, showing the discovery of the element. The first image shows the location, the University College London. The second, a drawing of the two discoverers, William Ramsay and Morris Travers. The last image shows the time in which it was discovered. I also wrote some of its characteristics on the side of the film; colorless, odorless and unreactive. I also added the boiling and melting points of the gas and showed that it is one of the noble gases.
Polonium, 84
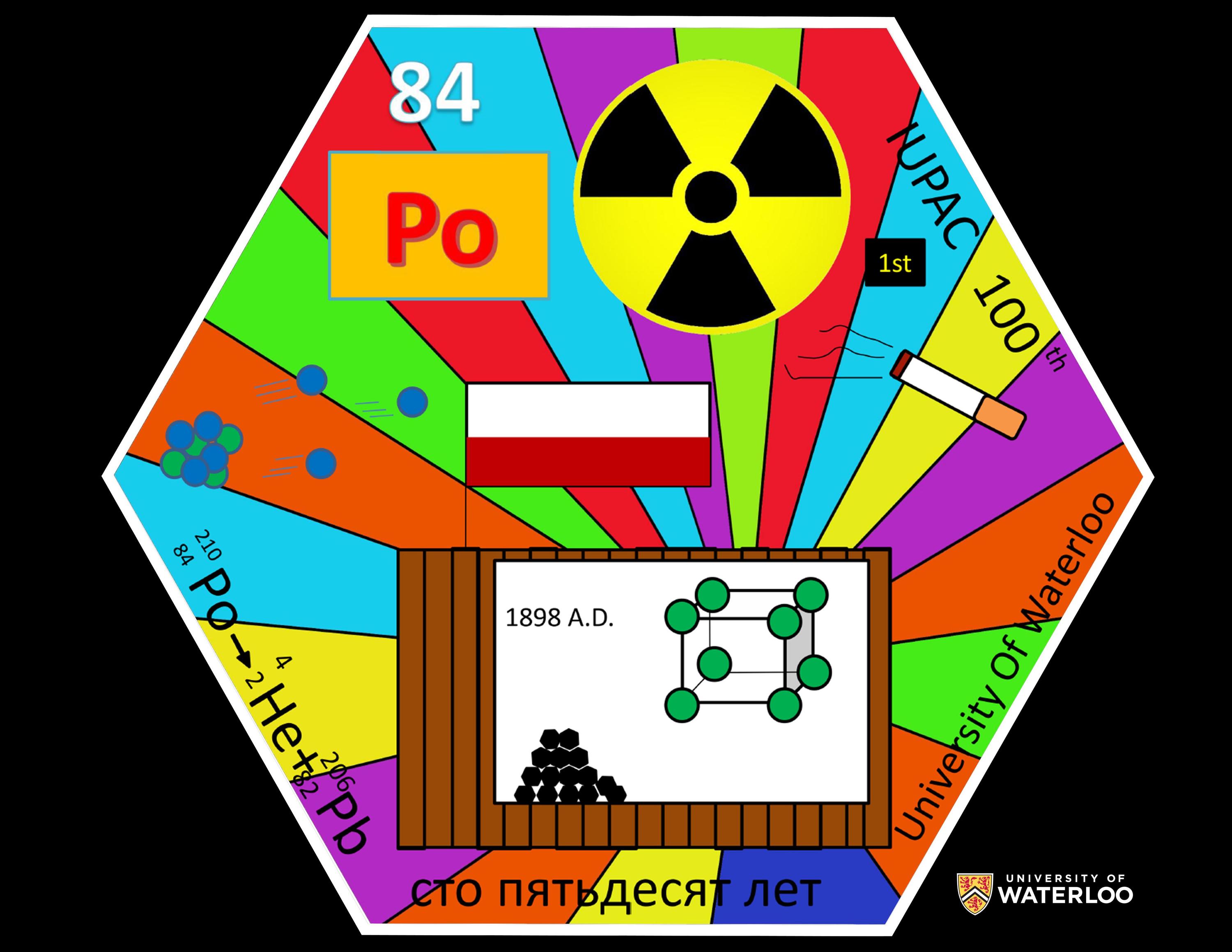
Waterloo, Ontario, Canada
Teacher: Rick Marta
Artist: Xingeng (Vincent) Zhao; representing the 2018 class of BJTU Nano students
1. In 1898, Polish scientist, Marie Curie, first isolated polonium (Po) by processing many tons of the radioactive uranium ore called pitchblende.
2. Po has a unique simple cubic crystal structure.
3. Po is the first naturally occurring radioactive element encountered in the periodic table. Po is an alpha-particle emitter.
4. To honour the 150th anniversary of Russian scientist Dmitri Mendeleev’s periodic table, 150 years is written in Russian.
5. The 100th anniversary of IUPAC is honoured.
6. Tobacco can contain trace amounts of Po; a cigarette is shown to advise of another health hazard associated with smoking.
Radium, 88

Calgary, Alberta, Canada
Teacher: Chris Lovallo
Artist: Alia McCracken, Casey Heirman
Students started with a Who-What-Where-When-Why questionnaire to guide their research into radium. Excerpts of ideas regarding how to express their research visually are below:
What? Radioactive symbol - Drawing of hand holding radium depicting cellular and DNA damage.
Who? Drawing of Marie and Pierre Curie - Portraits of people involved in discovery
Where? Drawing of radium mines or Ecole de Physique - Drawing of Radium Hot Springs
When? Quoting Marie Curie, “Radium is an element, it belongs to the people” - Drawing of Marie and Pierre’s radioactive papers
Why? Glow-in-the-dark watch - People using radium for everyday use.
Radon, 86
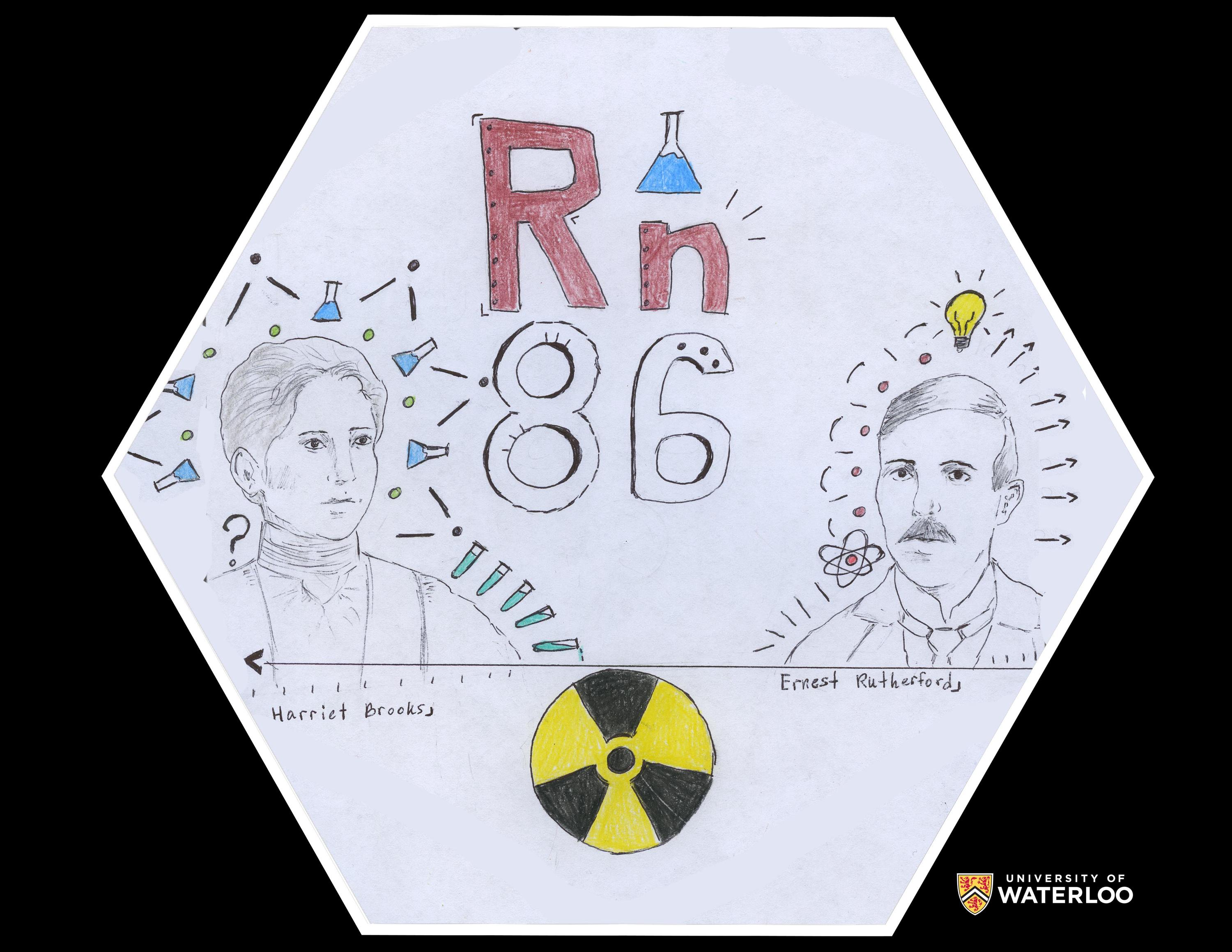
Cincinnati, Ohio, U.S.A.
Teacher: Michael Geyer
Artist: SHS Chemistry Club
The hand-drawn artwork on this tile is the result of a very small group of students from our school's Chemistry Club, and one artist from a Chemistry class at our school. It depicts the two scientists who discovered radon, namely Ernest Rutherford and his research assistant, Harriet Brooks at McGill University. The universal symbol for radiation is included due to the nature of this element.
Actinium, 89

Woodbridge, Ontario, Canada
Teacher: Adriana DiPietro
Artist: ECSS STEM Club
Actinium was discovered in 1899 by a French chemist named André-Louis Debierne. Debierne isolated actinium from a uranium-rich mineral ore. In 1902, a German chemist named Friedrich Oskar Giesel independently discovered the element. Since Debierne discovered the element first, he was the scientist who got to name it. Actinium is a radioactive substance that glows blue. It can be used in cancer treatment, but it can also cause damage to DNA. Actinium can also be used as a source of neutrons.
1905-1914: lutetium
Lutetium, 71

Kitchener, Ontario, Canada
Teacher: Panda Marsh
Artist: Laili Rohani
Lutetium is a chemical element with symbol Lu and atomic number 71. It is a silvery-white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted among the rare earth metals. The honour of discovering lutetium went to Georges Urbain at the Sorbonne in Paris, since he was the first to report it. Other chemists, namely Karl Auer in Germany and Charles James in the USA, having begun to isolate lutetium, were about to make the same discovery.
1915-1924: hafnium, protactinium
Hafnium, 72

Torquay, Queensland, Australia
Teacher: Stephanie Whitehead
Artist: Tahlia Brookes
I came up with the design for the element tile of hafnium by learning about the history of the element and its properties. Hafnium was discovered in 1923 by Georg von Hevesy and Dirk Coster in Hafnia now known as Copenhagen. The city is often recognized for its bright houses that lines the canals as shown in my artwork. Also I coloured these houses in rainbow colours because when hafnium oxidizes, it forms a rainbow-coloured surface.
Protactinium, 91
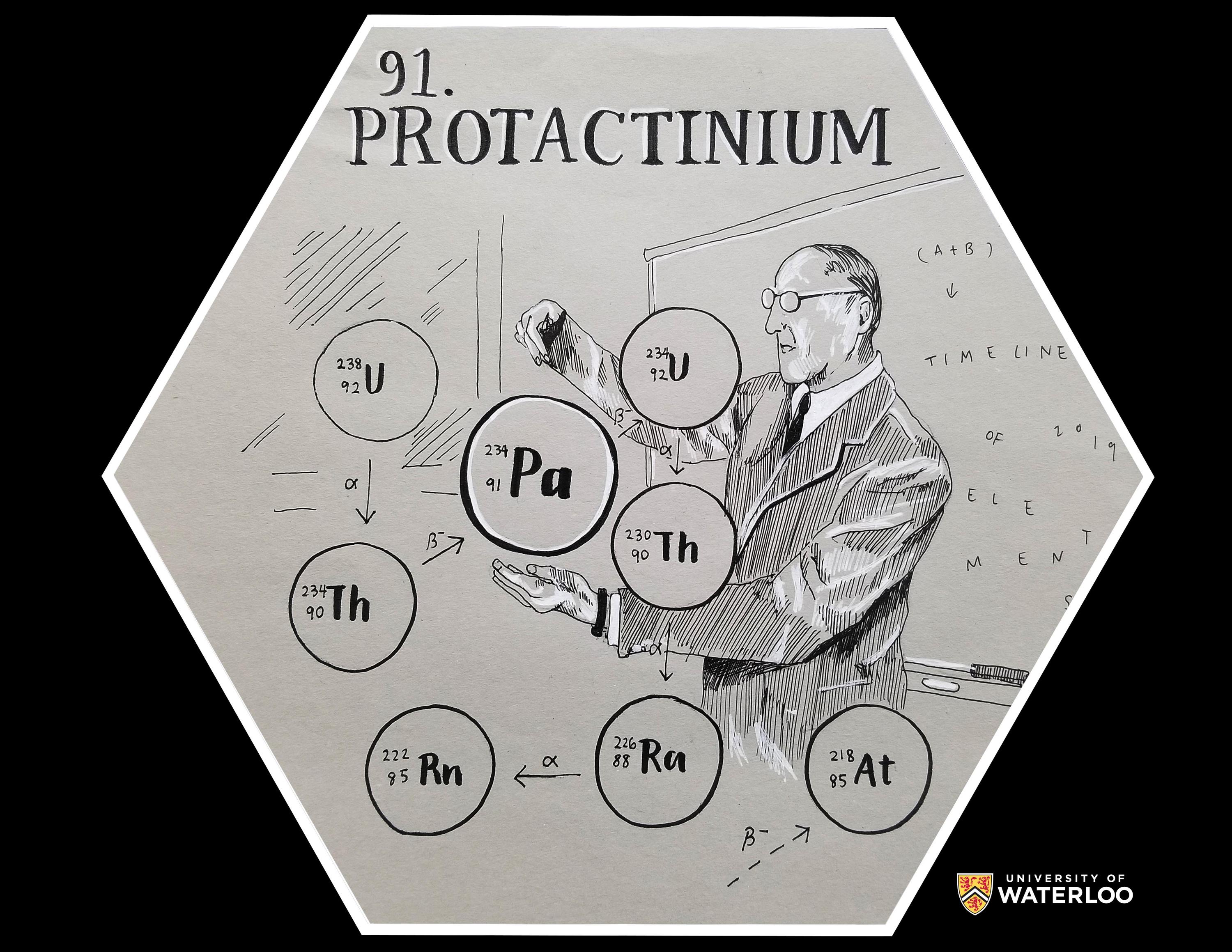
Plano, Texas, U.S.A.
Teacher: Nicole Lyssy
Artist: Emily Ren
I used a black micron and white gellyroll pen on toned paper to create my artwork. Protactinium was first identified in 1913 by Kasimir Fajans and O.H. Gohring while they were studying uranium's decay chain. The artwork depicts Kasimir Fajans surrounded by an enlarged uranium decay chain diagram. His hands surround the element Pa, which is the focus of the artwork. The background of the image has a chalkboard that subtlety says "timeline of elements 2019" as an additional touch to the artwork.
1925-1934: rhenium
Rhenium, 75

Okotoks, Alberta, Canada
Teacher: Debra Carlson
Artist: Jake Baverstock
The rhenium element design was created using a black & white negative and positive graphic design, overlaid onto a 1690 map of the Rhine Valley in Germany (Cartographer De Witt). The final production looks at the history of the element but depicts it in a modern stylized work. The three fountain pens represent each main discoverer of the element, and rhenium’s early use in pen tips. The stylized “Re” and compass rose work to balance the artwork and give a feeling of the time-period. The dashed lines divide the map from the graphic design.