From Hydrogenation to Alkylation: A Journey with Iron Complexes
Jean-Luc
Renaud
Professor
Université
de
Caen
Normandie
Laboratoire
de
Chimie
Moléculaire
et
Thioorganique
Caen,
France
Tuesday,
June
4,
2019
2:30
p.m.
C2-361
(Reading
Room)
Abstract: Economic
constraints
and
environmental
concerns
in
chemistry
have
led
to
increased
demand
for
the
replacement
of
noble
metals
used
in
chemical
processes
by
Earth-abundant
ones.
Iron-catalyzed
reduction
has
received
intensive
attention
and
some
iron
complexes
have
shown
activities
and
selectivities
that
are
competitive
with
those
of
noble
metals.[1]
However,
exchanging
noble
metals
for
cheap,
abundant,
and
biocompatible
iron
complexes
to
perform
reduction
is
not
the
sole
criterion
to
render
such
complexes
attractive
for
industrial
applications,
the
catalytic
activities
and
the
price
of
the
ligand
must
also
be
taken
into
account.[2]
In
our
ongoing
research
on
iron-catalyzed
reduction
and
oxidation,[3]
some
new
cyclopentadienone
iron
tricarbonyl
complexes
have
been
designed
based
on
a
"transition
metal
frustrated
Lewis
pair"
approach.[4]
Their
application
in
chemoselective
reduction
and
alkylation,
as
well
as
a
detailed
mechanistic
study
will
be
presented
(Scheme
1).[5]
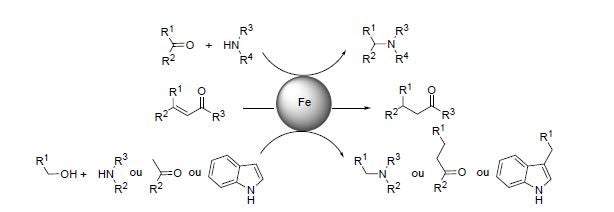
Scheme 1. Iron-catalyzed chemoselective reduction and alkylation reactions.
References
[1]
a)
I.
Bauer,
H.-J.
Knölker,
Chem.
Rev.
2015,
115,
3170-3387.
b)
D.
Wei,
C.
Darcel,
Chem.Rev.
2019,
119,
2550-2610.
c)
L.
Alig,
M.
Fritz,
S.
Schneider,
Chem.
Rev.
2019,
119,
2681-2751.
d)
D.
S.
Mérel,
M.-L.
Tran
Do,
S.
Gaillard,
P.
Dupau,
J.-L.
Renaud
Coord.
Chem.
Rev.
2015,
288,
50-68.
[2]
P.
Dupau,
M.-L.
Tran
Do,
S.
Gaillard,
J.-L.
Renaud,
Angew.
Chem.
Int.
Ed.
2014,
53,
1300
4-13006.
[3]
A.
Pagnoux-Ozherelyeva,
N.
Pannetier,
M.
D.
Mbaye,
S.
Gaillard,
J.-L.
Renaud,
Angew.
Chem.
Int.
Ed.
2012,
51,
4976-4980.
b)
A.
Lator,
S.
Gaillard,
A.
Poater,
J.-L.
Renaud,
Chem.
Eur.
J.
2018,
24,
5770-5774.
[4]
a)
T.-T.
Thai,
D.
S.
Mérel,
A.
Poater,
S.
Gaillard,
J.-L.
Renaud,
Chem.
Eur.
J.
2015,
21,
7066-7070.
b)
A.
Lator,
Q.
Gaignard
Gaillard,
D.
S.
Mérel,
J.-F.
Lohier,
S.
Gaillard,
A.
Poater,
J.-L.
Renaud,
J.
Org.
Chem.
2019,
84,
in
press.
[5]
a)
L.
Bettoni,
C.
Seck,
M.
D.
Mbaye,
S.
Gaillard,
J.-L.
Renaud,
Org.
Lett.
2019,
21,
3057-3061.
b)
A.
Lator,
S.
Gaillard,
A.
Poater,
J.-L.
Renaud,
Org.
Lett.
2018,
20,
5985-5990.
c)
C.
Seck,
M.
D.
Mbaye,
S.
Gaillard,
J.-L.
Renaud,
Adv.
Synth.
Catal
2018,
360,
4640-4645.
d)
C.
Seck,
M.
D.
Mbaye,
S.
Coufourier,
A.
Lator,
J.-F.
Lohier,
A.
Poater,
T.
R.
Ward,
S.
Gaillard,
J.-L.
Renaud,
ChemCatChem.
2017,
9,
4410-4416.