Chiral Iodoarenes and Iodanes for Transition Metal-free Asymmetric Reactions and Total Synthesis of Natural Products
Laurent
Pouységu
Professor,
SQuideau
Lab
Institut
des
Sciences
Moléculaires
(CNRS-UMR
5255)
Université
Bordeaux,
France
Wednesday,
April
24,
2019
2:00
p.m.
C2-361 (Reading
Room)
Abstract:
Over the last decade, the search for chiral iodoarenes and iodanes has been one of the most competitive area of investigations in hypervalent iodine chemistry, as these reagents represent a promising alternative to the use of (gradually rarefying) transition metals.[1] A wide number of chiral iodoarenes and iodanes have thus been described worldwide, and their evaluation in stereoselective (organoiodo-catalytic) transformations has led to a short list of truly efficient systems.[1]
In this context, our research team has been developing its own chiral iodocompounds based on structurally diverse biarylic (e.g., A and D), Salen-type (e.g., B and C) and helicenic (e.g., E) scaffolds.[2] These chiral reagents have mostly been successfully applied in asymmetric (and biomimetic) C–O bond-forming transition metal-free methodologies [e.g., the hydroxylative phenol dearomatization (HPD) reaction][3] and illustrated by the total synthesis of natural products, such as bisthymol, scyphostatin analogues, bacchopetiolone and maytenone.[2a-d,f] More recently, these chiral iodoarenes/iodanes have been investigated in more challenging stereoselective C–C bond-forming events.[2e]
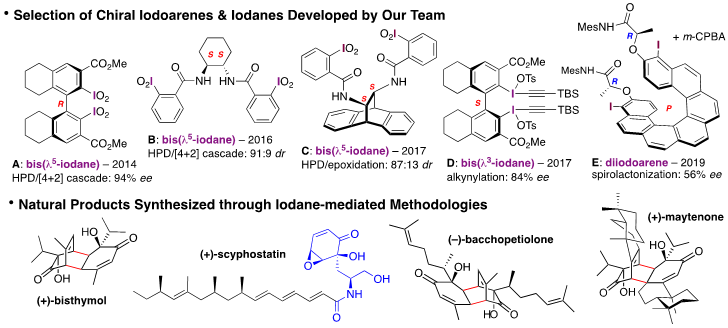
[1] Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328.
[2] Quideau, S.; Pouységu, L. et al.: a) Angew. Chem. Int. Ed. 2009, 48, 4605; b) Angew. Chem. Int. Ed. 2014, 53, 9860; c) Org. Lett. 2016, 18, 1120; d) J. Org. Chem. 2017, 82, 11816; e) Chem. Eur. J. 2017, 23, 2852; f) Chem. Eur. J. 2019, 25, 2852.
[3] Quideau, S.; Pouységu, L.; Peixoto, P. A.; Deffieux, D. In Topics Curr. Chem. 2016, Vol. 373 (In Hypervalent Iodine Chemistry; Ed.: T. Wirth), pp. 25-74; Springer.