(This is the first article in a four-part series)
Versions of a multi-part series of laboratories involving the synthesis and analysis of potassium tris(oxalato)ferrate(III) trihydrate (the “green crystal” labs) have been used in college-level general chemistry courses since at least the 1970s.1 The emerald-green crystalline complex can be analyzed by titrimetric2 and spectroscopic3 methods readily accessible to undergraduate laboratories. Our four-part implementation involves
- synthesis of K3Fe(C2O4)3•3H2O;4
- gravimetric analysis for H2O;
- acid-base titration for Fe and K and;
- redox titration for oxalate.
The sequence fits well in the current AP Chemistry course, accomplishing at least 6 of the 22 currently recommended laboratories in the curriculum. We use the labs as a capstone laboratory experience leading to the AP Chemistry exam. Our students routinely generate reliable results with the labs.
College Board will launch a new AP Chemistry curriculum in Fall 2013. As part of the change, a minimum of 6 of the 16 labs will use a guided inquiry format. Because College Board does not require all 16 labs to use a guided inquiry approach, these labs could have been kept in the existing format. However, we saw value in converting them to an inquiry approach, primarily because inquiry models authentic scientific investigation.
To ensure consistency with the AP Chemistry course revisions our conversions followed the Process Oriented Guided Inquiry Learning (POGIL) model for guided inquiry, which was also used by College Board when developing guided inquiry labs for the revised course.5 POGIL organizes lab experiments as follows: (1) The learning cycle starts with a question that leads to one or more key learning goals. (2) Concepts are developed from data. Here, it is important to note the distinction between development and confirmation: in development, analysis of the data leads to the concept, but in confirmation, the data merely affirm a concept already revealed by the instructor. (3) Strategically placed questions about the data guide students to the desired conceptual understanding. (4) After the student has developed the concept, it is applied to a different, albeit related, problem, through the use of post-lab questions, discussions or assignments.
For the entire four-lab sequence, the question to be investigated is, “What compound is formed when iron(III) chloride reacts with potassium oxalate in aqueous solution?” The learning goal is to distinguish transition metal complexes from simple ionic compounds. In this case, the reaction might be expected to produce a simple ionic compound with formula Fe2(C2O4)3; that the actual formula is quite different strongly suggests that a different way of thinking (i.e., complex formation) is in order. Moreover, the answer to the guiding question affords an opportunity to explore hydrates as a variety of crystalline solid.
Synthesis lab conversion
We converted Lab 1, the Synthesis, to a guided inquiry format by first identifying the critical features of the existing structured inquiry-style lab procedure and then creating a sequence of pre-lab questions that, when answered, would suggest to students a procedure that is essentially a recreation of the structured inquiry procedure. The difference, of course, is that responsibility for providing the procedure is relocated from instructor to student; the pre-lab questions and the instructor guide the way. The pre-lab questions blend consideration of topics (solubility and kinetics, for example) with bench-level decision making (such as deciding what glassware to use, and what volumes or masses of reactants could yield enough product to allow for analyses in the subsequent three labs.)
For example, in the original procedure,6 students were directed to
- Measure out 8.00 mL of 2.50 M FeCl3•6H2O into a graduated cylinder and pour it into a 50 mL beaker.
- Transfer 12 grams (recorded to the nearest thousandth of a gram) of K2C2O4•H2O into a 100 mL beaker, and dissolve the compound in approximately 20 mL of distilled water.
To guide students to a workable procedure, the pre-lab questions took the following form:
Pre-Lab Problem 1: If we use 8.00 mL of 2.50 M FeCl3•6H2O, how many grams of this compound do we have?
Expected answer:
0.00800 L x 2.50 moles/L x 270 g/mole = 5.40 g
Pre-Lab Problem 2: Will the reaction proceed faster by using solid K2C2O•H2O as the reagent or by dissolving it in water and using the solution as the reagent? Explain.
Expected Response: The reaction will take place much faster if K2C2O4•H2O is dissolved in water because the reactant particles are moving and there is much better mixing.
Subsequent essential features of the procedure, such as heating the solution and performing a recrystallization to purify the product, are likewise suggested to students through the pre-lab questions.
Pre-Lab Problem 3: Will the reaction take place faster if the solution is heated or if it reacts at room temperature?
Expected Response: The reaction will take place faster at the higher temperature because K2C2O4•H2O is more soluble at elevated temperatures and the reactant particles are moving faster. (Students can look up the solubility of potassium oxalate at different temperatures to verify this.)
Pre-Lab Problem 4: How can the product compound be precipitated out of solution?
Expected Response: The product compound can be precipitated out of solution by cooling it down in an ice bath, since it will be less soluble at the lower temperature. (Students can expect that at a higher temperature the product will be more soluble. Exceptions to this general rule are rare.)
Pre-Lab Problem 5: How can these crystals be purified?
Expected Response: They can be purified by dissolving them in hot water and allowing them to cool and recrystallize overnight at room temperature.
If students have not previously used recrystallization, a research assignment sends them to Wikipedia and Google for information about recrystallization as a common technique. In the assignment, students would assess the applicability of recrystallization and identify limitations to recrystallization that would bring additional purification steps — washing the crystals with ice water and acetone — into the proposed procedure. Questions about purification offer a window into the three analysis labs because questions considering the consequences of not purifying the crystals can be raised at the time the students are developing their lab procedures.
Following the pre-lab questions, students write a lab procedure that is submitted to the instructor for approval before beginning the lab. The procedure would be accompanied by a data table that reflects the goals of the lab. In this lab, the data table would include the masses of the reactants used and the mass of the purified product compound, in support of a percent yield calculation. Post-lab problems continue the data-driven approach by introducing additional data in the context of the completed lab procedure. For example, qualitative analysis is applied to the product:
Post-Lab Problem 1: Based on the reactants of this reaction, what are the possible components of the crystalline product of the reaction?
Expected Response: Iron(III) ions, chloride ions, potassium ions and oxalate ions (C2O42-) and even water.
Post-Lab Problem 2: Since the reaction is carried out in aqueous solution, is it possible that the product could be a hydrate compound?
Expected Response: Yes, that is a distinct possibility.
Post-Lab Problem 3: Two qualitative analysis procedures are carried out on samples of an aqueous solution of the purified green crystalline compound. A flame test on one of the samples shows the lavender flame of potassium. Silver nitrate is added to another sample of the compound solution, but no precipitate of silver chloride is obtained. What are the possible components of the compound?
Expected Response: K+, Fe3+, C2O42-(maybe), and H2O. The failure to observe a white precipitate means that free chloride ions and free oxalate ions are not present. Oxalate ions might be present if they are held rather tightly as part of a complex ion. Chloride ion could be held as part of a complex but because of the very low solubility of silver chloride, evidence of a precipitate would be expected if chloride ions were present, even in a complex.
Clearly, the pre-lab question approach to developing a lab procedure requires much more time than the old structured-inquiry approach required. But the AP Chemistry course redesign provides for the additional time required: the simultaneous reduction in labs from 22 to 16, while mandating six inquiry-based labs, strongly suggests that the inquiry labs are expected to take about twice as long as the old-style labs. The increased time investment facilitates much deeper, more thoughtful engagement with the lab’s design and the chemistry on which that design it is founded. Ultimately, deeper engagement with design provides students with an experience more like that of a researcher, and is consistent with the principle of “less breadth and more depth” that is driving the AP Chemistry course redesign.
The next installment in this series will explore conversion of the lab to determine the percent water of hydration by gravimetric analysis.
References
- D.W. Brooks, Journal of Chemical Education, page 218, Mar 1973.
- J. Olmsted, III, Journal of Chemical Education, pages 1031-1119, Dec 1984
- R.F. Dallinger, Journal of Chemical Education, 861-956, Oct 1995.
- CAS Registry No. 5936-11-8.
- AP Chemistry: Transitioning to Inquiry-Based Labs Workshop Handbook, College Board, 2012, pages 14-17.
- Green crystalline compound part 1: Synthesis. Southeast Missouri State University: Cape Girardeau MO 63701.
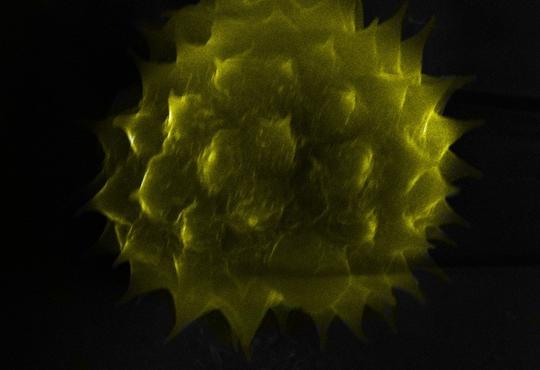
 This beautiful crystalline product (right) was synthesized in Pam Rush’s AP Chemistry class at The Charter School of Wilmington DE. It is 2.5 cm x 2.5 cm x 1 cm. [Photo taken by Sam Katz]
This beautiful crystalline product (right) was synthesized in Pam Rush’s AP Chemistry class at The Charter School of Wilmington DE. It is 2.5 cm x 2.5 cm x 1 cm. [Photo taken by Sam Katz]