Introduction
The use of natural gas (methane) and propane as fuel gases exemplifies numerous areas of general science and general chemistry: intermolecular forces, states of matter and changes of state; heats of vaporization; liquid–vapour equilibrium and vapour pressure; stoichiometry and heats of combustion; gas laws; carbon dioxide emissions and climate change. The chemistry and technology of using these gases as fuels can be used to connect these topics to a practical context. This article presents some selected physical and chemical properties of the two fuels and some basics of fuel gas use, working from the gas source or cylinder, to the gas supply pressure regulator, then to the burners. This material offers a variety of relevant applications along with ideas for interesting test questions.
Many engineering units are used in this area; I have usually converted these into more common units by using Professor Wildi’s Visualization of Units reference pages.1 Some engineering units are given along with the more common equivalents, e.g., inches of water with kPa and atm, and Btu/h with kW.
Physical and chemical properties
The Table contains properties relevant to the use of natural gas (methane) and propane as fuel gases. The data are taken from the CRC Handbook2 or Wikipedia.3 Although ‘natural gas’ and ‘propane’ are sometimes taken to be methane and propane respectively, the commercial products are not pure methane or propane; they are petrochemicals, and depending on the source and processing history, they contain varying small proportions of other alkanes of similar physical properties.3,4
Physical states and gas supply sources
The intermolecular forces between the very small molecules of methane (CH4) are very weak. Methane cannot be condensed to a liquid by pressure at ordinary temperatures because the critical temperature3 of methane is −82.1 ºC. Above this temperature no liquid phase exists. Natural gas, which is mostly methane, is bought and used by consumers as a gas from a pressurized natural gas distribution system or as a compressed gas in tanks. Natural gas can be liquefied at a pressure of 45.8 atm at or below its critical point temperature of −82.1 ºC to form liquefied natural gas (LNG)3 for commercial transport.
Table: Selected physical and chemical properties
| Property | Methane | Propane |
| Chemical formula | CH4 | C3H8 |
| Normal boiling point (°C) | − 164 | − 42.1 |
| Critical point (T °C, P atm) | − 82.1, 45.8 | 96.8, 42 |
| Density of liquid (kg/L) | --- | 0.51 |
| Flammability limits (%) | 4.7 – 15 | 2.2 – 9.6 |
| Ignition temperature (°C) | 482 – 632 | 493 – 604 |
| Flame temperature (°C) | 1950 | 1980 |
| Heat of vaporization (kJ/mol) | --- | 20.1 |
| Heat of combustion (MJ/mol, kJ/g) (product water as a gas) |
0.805, 50.00 | 2.044, 46.35 |
Relative to methane, the intermolecular forces between the larger molecules of propane (C3H8) are greater. The critical point of propane, above which no liquid phase exists, is above any temperature normally encountered. Propane is bought and used domestically as a volatile, pressurized liquid, called propane or liquefied petroleum gas (LPG)3, and is dispensed under pressure into cylinders, tanks, or vehicle tanks.
The propane liquid – vapour equilibrium
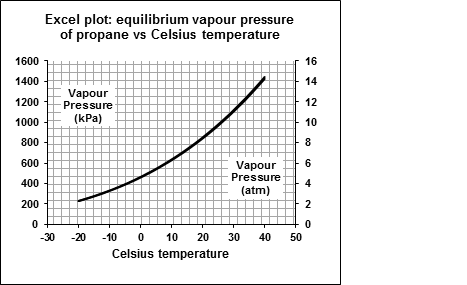
Propane in a cylinder or tank at ordinary temperatures is a highly volatile liquid in equilibrium with its vapour under a pressure — the equilibrium vapour pressure,3 which varies with temperature as shown in the Excel® plot (next page). This curve describes a function known as the Clausius-Clapeyron equation.3 The 1974 edition of the CRC Handbook5 gives the required equation and coefficient data to calculate values of the vapour pressure at any temperature, in mm Hg units.
Excel functions were used to calculate the vapour pressure at every integral temperature from −20 ºC to +40 ºC, and to convert the values to both atm and kPa units. These values were plotted with the two pressure units shown as the two scales on the plot. The values rise from about 2 atm at −20 ºC to above 14 atm at +40 ºC. A propane cylinder in the sun on a hot summer day will have an internal pressure well above 10 atm.
The evaporation of liquid propane is endothermic. As propane vapour is drawn from a cylinder to be burned in a grill, the pressure drops and liquid propane vaporizes according to Le Châtelier’s principle3 to restore equilibrium. This requires heat energy, which must flow in from the surroundings. The temperature of the liquid drops as this happens because heat flow from the surroundings is not instantaneous; the temperature of the liquid propane does drop significantly while grilling:
C3H8 (l) + 20.1 kJ ⇄ C3H8 (g)
Propane cylinders
 A common propane cylinder type used for grills is the ‘20 lb’ cylinder. The valve guard of the cylinder is stamped with various data, including the tare mass 8.4 kg (18.5 lb), the water volume 21.6 L (5.7 US gal) and the date of manufacture.6 The maximum fill volume allowed is about 85% of the water volume, or about 18.4 L, which translates as about 9.4 kg of propane, or about 20.6 lb. Cylinders are filled by mass to avoid overfilling, hence the term ‘20 lb’ cylinder.
A common propane cylinder type used for grills is the ‘20 lb’ cylinder. The valve guard of the cylinder is stamped with various data, including the tare mass 8.4 kg (18.5 lb), the water volume 21.6 L (5.7 US gal) and the date of manufacture.6 The maximum fill volume allowed is about 85% of the water volume, or about 18.4 L, which translates as about 9.4 kg of propane, or about 20.6 lb. Cylinders are filled by mass to avoid overfilling, hence the term ‘20 lb’ cylinder.
Overfilling greatly increases the possibility of dispensing liquid propane when the valve is opened. In addition to the hazard of fire or explosion, liquid propane evaporates so rapidly in contact with skin that it is a severe frostbite hazard. To avoid this, all cylinders sold now must be fitted with a valve with an OPD or overfill protection device which closes the valve when the liquid propane reaches a set level in the cylinder.7 Cylinders must be secured upright when used.
Cylinders may be used for 10 years from the date stamped on the guard ring, after which time they may be re-qualified by a visual inspection for a further 10 years.6
Gas supply pressure regulation
 The most common gas pressure regulator system is the diaphragm + spring type.8 This is a very rugged and dependable regulator that uses the movement of a diaphragm to open or close a valve. When there is excess pressure in the gas line to the burners, the regulator valve shuts, and when the pressure in the supply line drops too low, the regulator valve opens. In this way, through negative feedback,3 the pressure of fuel gas flowing to the burners is maintained at the specified gauge pressure above atmospheric pressure. A normal gauge pressure for natural gas burners is 7 inches of water (1.7 kPa, 0.017 atm)9 and a normal gauge pressure for propane burners is 10.5 inches of water
The most common gas pressure regulator system is the diaphragm + spring type.8 This is a very rugged and dependable regulator that uses the movement of a diaphragm to open or close a valve. When there is excess pressure in the gas line to the burners, the regulator valve shuts, and when the pressure in the supply line drops too low, the regulator valve opens. In this way, through negative feedback,3 the pressure of fuel gas flowing to the burners is maintained at the specified gauge pressure above atmospheric pressure. A normal gauge pressure for natural gas burners is 7 inches of water (1.7 kPa, 0.017 atm)9 and a normal gauge pressure for propane burners is 10.5 inches of water
(2.6 kPa, 0.026 atm).10
Notice that when a propane cylinder runs out of propane, the regulator valve will be fully open, and air and water vapour will be able to enter the cylinder, possibly causing corrosion or freezing to block the regulator. To prevent this, the cylinder valve must be closed if the propane is exhausted.
Propane and natural gas burners
 The control and burner assembly of a grill is designed to control the fuel gas flow and mix the fuel gas with an appropriate amount of air, within the flammability limits,3 such that the mixture can be easily ignited and completely burned in safety. Resistance to blowing out in a gusty wind is also a consideration. The air is drawn into the burner by a carburetor effect3 based on Bernoulli’s principle.3
The control and burner assembly of a grill is designed to control the fuel gas flow and mix the fuel gas with an appropriate amount of air, within the flammability limits,3 such that the mixture can be easily ignited and completely burned in safety. Resistance to blowing out in a gusty wind is also a consideration. The air is drawn into the burner by a carburetor effect3 based on Bernoulli’s principle.3
CH4 + 2 O2 → CO2 + 2 H2O + 0.805 MJ
C3H8 + 5 O2 → 3 CO2 + 4 H2O + 2.044 MJ
Considering the balanced thermochemical equations for complete combustion, we see that to get the same amount of heat energy from combustion, it is necessary to burn more moles of methane than of propane. By proportion:
2.54 CH4 + 5.08 O2 → 2.54 CO2 + 5.08 H2O + 2.044 MJ
To achieve the same heat energy output, a natural gas burner must use 2.54 times the number of moles of fuel gas as a propane burner. It does, however, release less carbon dioxide into the atmosphere. According to Avogadro’s law,3 if the fuel gases are at the same temperature and pressure, the natural gas burner will require 2.54 times the fuel gas flow rate as the propane burner.
So natural gas grills require supply tubing with larger flow capacity than propane grills, and the burner controls and burner design may be different. Conversion of a grill from propane fuel to natural gas fuel is not a simple matter.
Burn rate of a propane grill
In North America gas appliances, furnaces, grills and air conditioners are rated in the unit Btu/h,* e.g., Weber E310 grill rated at 38,000 Btu/h.11 What is the burn rate (g/s) of propane required to produce 10,000** Btu/h (2.93 kW)? In cooking, the product water is a gas. The appropriate value from the Table is 46.35 kJ/g of propane. The production of 2.93 kW (2.93 kJ/s) requires the combustion of 0.0632 g/s of propane.
* British thermal unit per hour
** The value of 10,000 Btu/h is an approximate amount to heat a propane grill. It is also a nice round number for students.
How long does a full ‘20 lb’ cylinder (9.4 kg) of propane burn at this rate? Answer: 149,000 s = 41.3 h. Every user of propane will have a different pattern of consumption, so this value is just an approximate guideline.
How to determine when to refill a propane cylinder
There are at least four methods of determining when to refill your propane cylinder, or to otherwise avoid running out of fuel at an inopportune moment. These are discussed in my article posted online.12
Questions for students
- A high efficiency gas furnace increases the recovery of combustion heat energy by using two stages of heat exchange, cooling the product gases to as low a temperature as possible. The first exchanger heats the indoor air, and the second exchanger heats the outside air going to the burner. As a result, the water vapour in the combustion gases gives up heat energy, condensing to liquid water which flows to a drain or a sump pump.
Calculate the percentage increase in heat energy obtained by condensing relative to not condensing for (a) natural gas furnaces and (b) propane furnaces. Hint: write the two combustion equations on page 23 showing the physical states of the reactants and products (all gases). Now write them again with the product water as a liquid, and calculate the new heat energy value for each equation. Don’t forget to look for the number of moles of water produced in order to help determine the heat released as the water is condensed. The heat of vaporization of water is 40.65 kJ/mol.3 The percentage increase in energy obtained is significant to energy efficiency in today's world.
Answers: (a) 10.1% (b) 8.0%
- What is the theoretical minimum temperature at one atmosphere pressure at which a propane grill can be operated? Explain your answer. In practice, the burners may not actually receive gas rapidly enough to sustain a flame, nor may the burners be hot enough to be useful.
Answer: Just above the normal boiling point temperature of -42.1 ºC, where the propane pressure, in theory, will be enough to cause it to flow through the regulator.
- Use the Excel plot above to roughly estimate the vapour pressure of propane in a 20 lb cylinder at (a) 25 ºC and (b) 0 ºC. Now suppose the liquid propane has just run out at each temperature. How long will the grill burn in each case at 10,000 Btu/h? Assume that the grill will continue to function until the propane pressure drops to 1 atm pressure. Use the ideal gas law3 as an approximation to determine how much propane is in the cylinder in each case. These answers will be very approximate.
Answers:
- 9.7 atm, 340 g, 90 min
- 4.6 atm, 150 g, 40 min
References (websites accessed November 2014)
- Theodore Wildi: http://www.wildi-theo.com; www.wildi-theo.com/index.php?p=SI_Prefixes.
- CRC Handbook, CRC Press, 54th edition, 1973-74.
- www.wikipedia.org for: propane; natural gas; methane; critical point (thermodynamics); vapour pressure; Clausius-Clapeyron; Le Châtelier’s principle; gas cylinder; pressure regulator; gas burner; heat of combustion; Avogadro’s law; carburetor; Bernoulli’s principle; liquefied natural gas (LNG); liquefied petroleum gas (LPG); gas laws; negative feedback; flammability limits; water.
- Engineering Toolbox: www.engineeringtoolbox.com/chemical-composition-gaseous-fuels-d_1142.htm
Union Gas: www.uniongas.com/aboutus/aboutng/composition.asp - CRC Handbook, CRC Press, 54th edition, 1973-74, Table page D241.
- Canadian Propane Association: www.propane.ca/sites/default/files/files/Cylinder%20Markings_En(1).pdf
- Propane101: www.propane101.com/opdcylindervalves.htm
- Emerson Process Management: www.documentation.emersonprocess.com/groups/public/documents/reference/d351798x012_05.pdf
- Dormont Regulators: http://0323c7c.netsolhost.com/docs/regulatorspecsheet.pdf
- Gas Hoses and Regulators: http://gashosesandregulators.com/propaneregulatorfacts.html
- Weber Inc.: www.weber.com/explore/grills/genesis-series/genesis-e-310
- D. Cash, Using a Pressure Gauge with a Propane Cylinder: www.uclmail.net/users/dn.cash/Propane.pdf.







