Introduction
Tea:1 How to brew it, how to clean a teapot, and much more — the subject of an article in our recipe file. The ideas presented in the article (box below) relate to general chemistry, general physics, and plant biochemistry and could be useful in teaching general chemistry. The numbers in red have been added to indicate points of interest for discussion later in this article. We were initially interested in the method of cleaning stains from teapots (and coffee carafes). These stains are caused by the tannins1 present in these beverages. First, a note on tannins. Then, brief notes on the other points of interest.
(Newspaper clipping: Byline, Source, and Date: Missing)
Perfect Tea
“If tea is your preferred beverage, Dr. Samuel Twining,[*] director of England’s world renowned Twining Tea Company, offers some valuable advice on brewing a superior cuppa.
First, the teapot must be chosen with care. Asked what material makes the best teapot, Twining warns against aluminum [1] and instead recommends earthenware, silver, glass or stainless steel.
To brew the tea, “start by filling an empty kettle with fresh cold water drawn from the cold tap. The reason is that cold water is full of oxygen, and tea loves oxygen.” [2]
Knowing when to add the water is crucial, Twining explains. Once you’ve warmed the pot and put in the tea of your choice, “you should add the water the moment it comes to the boil.” Don’t leave it at a rolling boil or all the oxygen will be pumped out of the water [3] and the result will be “flat, dull, uninteresting tasting tea.” [4]
What about brewing time? [5] Twining says that small-leaf teas, such as English and Irish breakfast, take about three minutes to brew; the medium-leaf teas, such as Earl Grey, about five; and the large-leaf teas, such as Lapsang souchong, jasmine and Darjeeling, will take up to seven minutes. [6] “And because they take a long time to brew” he adds, “it is not inelegant to take the lid off the teapot and stir with a spoon [7] to get an even brew throughout before you serve it.”
Twining closes with a word on cleaning teapots. “The trick to keeping your teapot clean is to never use a detergent [8] but to use what we call bicarbonate of soda [baking soda in North America]. Two dessertspoonfuls [9] in some hot water will gently clean your teapot without leaving a stain [10] or an aftertaste.” [11]
[*] Samuel Twining is now retired.2
Tannins
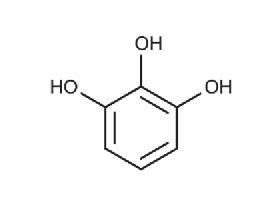
The tannins are a subgroup of the polyphenols,1 a very populous group of chemical substances found in plants. Tea leaves and coffee beans are very rich in polyphenols, including tannins. Tannins impart a characteristically bitter, astringent taste to beverages. Tannins from red grape skins are a very important element in the taste of fine red wines. The term tannin derives from the use of oak-bark-derived chemicals in the tanning of leather (German: tannenbaum = oak or fir tree). The Wikipedia article on ‘phenolic content in tea’1 states that the catechin1 polyphenols alone make up 25% of the dry mass of tea leaves. The polyphenol gallic acid1 is incorporated into the structures of many other more complex polyphenols, such as epigallocatechin,1 epigallocatechin gallate, and tannic acid.1 Tannic acid is not a single molecular structure, but a family of mono- and poly-esters of β–D-glucose1 with 2 to 12 gallic acid residues. With the central glucose in its more stable chair configuration, the five substituent groups project from equatorial bonds. The molecule has the flat disk-like shape of a six-bladed fan, with one missing blade. All structures below are taken from Wikipedia articles.
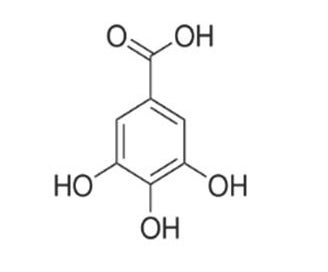 Gallic acid
Gallic acid
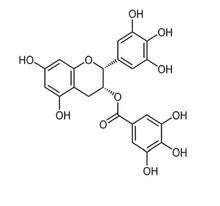
Epigallactechin Gallate
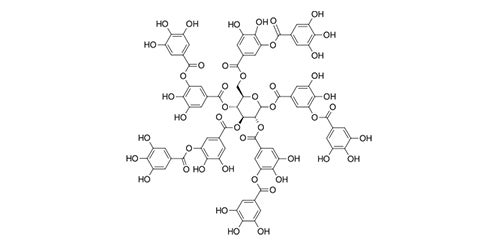 Tannic acid
Tannic acid
“Tannins are present in the leaves, bark, fruits and seeds of many plants. The main function of these compounds is to provide protection against microbial pathogens, harmful insects and other herbivores.”3 Water-soluble tannins including tannic acid bind to and co-precipitate with many classes of organic compound, e.g., water-soluble globular milk proteins.1 After extraction by water (brewing) and interaction with protein or other components of the beverage, a precipitate forms on the inner surface of the containment vessel. This precipitate is not easily removed from earthenware,1 stoneware,1 or glass surfaces by hand washing with mild detergent and gradually builds up.
The tannin content of tea leaves and both green and roasted coffee beans has been assayed.4 Dry tea leaves contained 37 ± 2.6 mg g−1 by mass tannic acid equivalents as analyzed by spectrophotometry and 24 ± 2.8 mg g−1 by an albumin1 protein precipitation technique. Green coffee beans contained 6.6 ± 0.6 mg g−1 by mass tannic acid equivalents as found by protein precipitation or 6.8 ± 2.3 mg g−1 by spectrophotometry. The same figures for roasted beans were 18 ± 1.7 and 17 ± 2.7 mg g−1, respectively. Typically, dry tea leaves therefore contain about 3% by mass of tannins, and dry roasted coffee beans about 2% by mass.
Chemicals in tea
1,2-Benzoquinone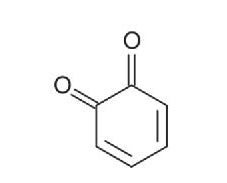
Tea is produced from the plants Camellia sinensis var. sinensis and C. sinensis var. assamica. The tender new leaves are harvested and processed, either as large leaf fragments with little air oxidation (green tea – 20%) or as small leaf fragments with considerable air oxidation (black tea – 78%). Brewed black teas contain, amongst other compounds, amino acids, alkaloids (e.g., caffeine), polyphenols (e.g., tannins), and theaflavins. The latter quinones1 and quinone polymers are produced by the air oxidation of polyphenols in the tea leaves catalyzed by the enzymes polyphenol oxidase and polyphenol peroxidase present in the leaves. Some products of these oxidation reactions are bitter (Wikipedia – ‘phenolic content in tea’) and darken the colour. ‘Black tea’ is a description of both the colour of the oxidized leaves and the colour of the resulting beverage.5
Brewing and drinking tea
The water temperature and brewing time of tea varies with the type of tea and the local custom. Black tea is usually brewed with water as hot as possible. Many of the taste components will not extract below 90 ⁰C (Wikipedia — ‘tea’). The polyphenols in tea and coffee are very weak acids, which contributes to the taste of the beverage. Conceivably, the addition of milk (2% — 4% fat) or cream (10% fat) modulates the taste of tea or coffee because the milk protein buffers the polyphenol acidity. Or perhaps the polyphenols and quinones are more soluble in the fat droplets than in the aqueous phase, reducing the perceived astringent taste.
The co-precipitation of milk casein1 proteins with tannins probably explains why a stoneware mug used to brew and drink tea with milk develops a particularly heavy, hard to remove deposit over time. The neutralization of the acidic tannins using sodium bicarbonate may be the key to Samuel Twining’s cleaning method (see Question 1).
Notes
[1] Excess aluminum in the diet may harm health.6 Aluminum metal cookware is known to leach aluminum ions into food, particularly if the food is acidic and cooked at high temperature.7 Both conditions occur in the brewing of tea, so avoiding aluminum teapots is sound advice from a health perspective. Aluminum ions may well alter the taste of the tea as well, although it would contravene medical ethics1 to ask a tasting panel1 to assess this.
[2, 3, and 4] These sentences are about the solubility of atmospheric gases in water,1,8 pressure dependence of solubility (Henry’s Law),1,8 temperature variation of solubility1,8 and tap-water aeration.1,8 “Tea loves oxygen” and “all the oxygen will be pumped out of the water” are examples of anthropomorphism.1,9
 Pyrogallol
Pyrogallol
The idea that oxygen-less water makes an inferior cup of tea requires study. Polyphenols, including tannins, are very reactive to oxygen.10 Pyrogallol,1 derived from gallic acid, was formerly used by chemists to remove traces of oxygen from compressed nitrogen. Trace oxygen may
cause a change in the taste of the tea by converting some polyphenols to more bitter quinones. On the other hand, the assumption that newly boiled water contains more dissolved oxygen than re-boiled water is questionable11 (see Question 4).
[5, 6, and 7] Brewing tea (and coffee) is solvent extraction by hot water followed by filtration.1 Hot water extracts soluble solids from the tea leaves and the undissolved residues are separated by filtration or by allowing the tea leaves to settle. The physical processes are movement by capillary action1 of water molecules into the tea leaves, and movement by diffusion of dissolved molecules out of the tea leaves, as well as by convection (bulk movement or advection)1 if the brew is stirred. Stirring of the tea is definitely required to ensure that the brew is uniform before pouring out. The larger the tea leaf fragments, the less relative surface area, and the greater the distances for diffusion, hence the longer brewing time required for larger-leaf teas.
[8 and 11] It is believed by wine and tea connoisseurs that detergent residues leave an aftertaste in the wine or the tea.12 Food safety is also an issue, if a tea pot is left without adequate cleaning for long periods.
[9] The dessertspoon is a recognized unit of volume in food preparation. In most jurisdictions, a dessertspoonful is equal in volume to two teaspoonfuls. A tablespoonful is equal to three teaspoonfuls. Simple eh? But there are at least four definitions of a teaspoon volume: metric
(5 mL); US (4.93 mL); CAN (4.74 mL); UK (5.92 mL)!!13
[10] This method does work. We used a tablespoonful of sodium hydrogen carbonate in each of a lightly stained glass coffee carafe and two extremely heavily stained stoneware tea mugs. The mugs were used daily to both brew a strong cup of tea and drink the tea with sugar and milk. The black-brown surface deposits had been impossible to remove with hand-washing detergent and a dishcloth. We added almost boiling water to the sodium hydrogen carbonate, waited for 30 minutes, and then poured out the liquid. Most of each deposit was visibly loosened, and the remainder of the deposit was easily removed by gentle rubbing with a wet paper towel. My hypothesis is that the tannins bind to other molecules by hydrogen bonding,1 and that this bonding is disrupted by converting the tannic acid molecules into tannate anions (see Question 1 below).
Questions for students
(a) Write a balanced net-ionic equation for the acid-base reaction in hot water.
[HT(aq) + HCO31-(aq) → T1-(aq) + H2O + CO2(g)]
(b) The reaction of a weak acid with a weak base is usually an equilibrium system that does not go 100% to completion unless there are special circumstances driving the reaction forward. What special circumstance drives this reaction forward?
**2. My English Breakfast Tea teabag contains 3.1 g of tea leaves (3.0% tannins by mass). Assume the tannins are a monoprotic acid HT (average molar mass of 1000 g/mol).
(a) Calculate the moles of HT present in the tea and the moles of CO2 produced by complete reaction of the tannins in one teabag with NaHCO3.
[both 9.3 x 10-5 mol]
(b) Convert this amount of CO2 into a volume of gas at 100 kPa and 21 ⁰C. Assume ideal gas behavior. [2.3 mL]
3. Aluminum atoms are very reactive to oxidation, but Al metal is protected by a thin adherent coating of Al2O3 preventing further reaction. Aluminum metal or foil may be used for cooking food, but there is a danger if the food is acidic, since the acid can dissolve the coating. The bare aluminum metal may then be dissolved by the acid.
(a) Write a balanced net ionic equation for tannin as a monoprotic acid HT reacting with Al2O3 in the presence of water.
[Al2O3 (s) + 6 HT(aq) →
2 Al3+(aq) + 6 T1-(aq) + 3 H2O]
(b) Write a balanced net ionic equation for tannin as a monoprotic acid HT reacting with Al metal in the presence of water.
[2 Al(s) + 6 HT(aq) → 2 Al3+(aq) + 6 T1-(aq) + 3 H2(g)]
4. The idea that “tea loves oxygen” and that dissolved oxygen improves the flavor of tea is pervasive on the web.
(a) Search for the specifications and price of an oxygen sensor capable of measuring the oxygen content of hot water (e.g., brewed tea). Such sensors are needed in the boiler industry because oxygen in the water corrodes boilers and must be carefully controlled.
(b) Design experiments to oxygenate tea as it brews and to determine the effect of dissolved oxygen on the taste of tea.
5. Post-operative instruction after a tooth extraction or gum surgery: “If bleeding continues or reoccurs, wrap a moist teabag in gauze and hold it firmly against the affected tissue until bleeding ceases.” Explain why and how this would be an effective method to halt bleeding.
References
- Wikipedia (www.wikipedia.com) for: tea; tannins; polyphenols; phenolic content in tea; gallic acid; epigallocatechin; tannic acid; glucose; globular protein; earthenware; stoneware; albumin; casein; medical ethics; faucet aerator; oxygen (physical properties); Henry’s Law; anthropomorphism; pyrogallol; filtration; diffusion; convection; advection; hydrogen bond.
- Time to take tea with the Twinings, Island Life Magazine
- Tannins — What do they represent in plant life? 2011.
- Tannin content of tea and coffee, Journal of Applied Toxicology, 1992, Wiley Books (abstract only).
- Black tea: chemical analysis and stability, RSC (Royal Society of Chemistry) Publishing, 2013 .
- Public Health Statement — Aluminum CAS #7429-90-5, Agency for Toxic Substances and Disease Registry, US Centers for Disease Control and Prevention.
- Risk Assessment of Using Aluminum Foil in Food Preparation, International Journal of Electrochemical Science 2012.
- Solubilities of oxygen and air in water, Engineering Toolbox:
www.engineeringtoolbox.com/oxygen-solubility-water-d_841.html; www.engineeringtoolbox.com/air-solubility-water-d_639.html. - Anthropomorphisms, Electronic Journal of Science Education, 2011:
- Tannin Chemistry: Techniques, Tannin Benefits, Winemaker Magazine, 2011.
- Destructive Myths: The Dissolved Oxygen Hypothesis, 2013.
- How to clean wine glasses, WikiHow: .
- Unit Conversions of Volume
Postscript: demonstrations and experiments with tea
A. Sodium hydrogen carbonate and tea in cold and hot water.
The chemistry of NaHCO3 with respect to the acids in tea varies according to whether cold or hot water is used. There could be an interesting experiment or demonstration here.
In cold water (room temperature) NaHCO3 dissolves and reacts with acid as described in Question 1 above. Place a tablespoonful of NaHCO3 powder in a small beaker and add 100 mL of cold water. Stir. Some of the powder dissolves. No bubbles are seen. Add a teabag of black tea. Tea slowly extracts into the solution. Bubbles of gas are seen.
In recently boiled hot water, a different result occurs. Adding hot water to the NaHCO3 powder causes a rapid evolution of gas. Hydrogen carbonate ion is unstable above 50 ⁰C, decomposing into water, carbon dioxide, and carbonate ion. The carbonate ion is a much stronger weak base than is hydrogen carbonate ion. Let the bubbling subside, and add a teabag of black tea. Tea extracts rapidly into the solution, rapid gas evolution recommences, and the teabag inflates in a balloon-like manner. Students can be asked to write balanced equations for the two steps of the reaction. They can also be asked to show that the two schemes produce identical amounts of acid neutralization overall. It is this latter scheme that describes the cleaning of a teapot.
B. pH measurement and total acid titration of tea.
If a pH meter is available, it would be an interesting exercise to prepare various kinds of tea, then measure the pH of each.
A titration against NaOH solution (weak acid – strong base reaction) using the pH meter to indicate the end point, at about pH 9, would allow the estimation of total acid content of each tea. Various teas could then be compared to soft drinks (e,g., colas) and fruit juices.







