The Canadian Chemistry Contest (CCC) consists of two parts: for Part A, students must answer 25 multiple choice questions and for Part B, students must write two essays from among three topics. It is designed to challenge top chemistry students in Canada in Thermodynamics, Kinetics, Equilibrium, Electrochemistry, Chemical Structures, Stoichiometry, the Gas Laws, Safety, Organic Chemistry, Periodic Trends, Acids, Bases and Solutions. High school teachers and university professors from across Canada are involved in creating the contest questions — see the acknowledgements to the 2016 question creators at the end of this article. The 2007–2016 exams and answer keys are available at www.cheminst.ca/outreach/canadian-chemistry-contest.
Designing multiple choice questions for students across Canada is challenging because the grade 11, 12 and cégep curricula are quite different from province to province. Question designers must create questions that are not just difficult or tricky but that will distinguish between the very best students and those who do not know the material as well. It is a common misconception that difficult questions discriminate strong students from weak students better than easy questions. Easy questions are often the best discriminators.1
In Part 1, I would like to discuss four questions on Part A of the 2016 CCC exam that had a discriminating index between 0.60 and 0.64. The discriminating index compares the performance of the students who scored in the top 27% of CCC to those who scored in the bottom 27%. The discriminating index can range from -1, where all of the students in the top 27% get the answer incorrect and the bottom 27% get the answer correct, to 1, where all students in the top 27% got the answer correct and all students in the bottom 27% got the answer incorrect. Best practices in multiple choice questions indicate that discriminating indices of greater than 0.3 are good and those with discriminating indices greater than 0.4 are excellent.2 Assessing the quality of multiple choice questions should consider the discriminating index, the difficulty index and the structure, which I will explore in more detail in part 2 in an upcoming issue of Chem 13 News.
In Part 2 I will also discuss an interesting finding from the exam: the question that was the most difficult, as measured by the lowest correct answers, was also the worst discriminator. I was surprised by how badly students did on this question but also inspired to gain a deeper understanding of student misconceptions by analysis of the CCC results.
Questions and analysis
- A 0.48 g piece of magnesium metal is placed in hydrochloric acid. Assuming the hydrochloric acid is in excess and the magnesium reacts completely how many grams of hydrogen gas are produced?
- 0.010 g
- 0.040 g
- 0.080 g
- 0.48 g
- 0.96 g
The second question on the exam, with a discriminating index of 0.63, was a relatively easy stoichiometry question with a given excess reagent. Only 63% of the students who took the exam answered it correctly.
- A student performs the following reaction to make solid sulfur:
Na2S2O3(aq) + 2HCl(aq) → 2NaCl(aq) + S(s) + SO2(g) + H2O(l)
The student records the following data at the start of the reaction:
| Concentration (mol L–1) | Volume (mL) | |
|---|---|---|
| Na2S2O3(aq) | 0.45 | 130 |
| HCl(aq) | 0.15 | 400 |
If the student recovers 0.89 g of solid sulfur from the experiment, what is the % yield of the reaction?
- 46%
- 48%
- 75%
- 89%
- 93%
This question on the exam was a little more of a challenging stoichiometric question. It provided the balanced chemical reaction, but the student had to determine the limiting reagent. Only 44% of the students got this question correct, and the discriminating index was 0.64.
- The metric known as reaction mass efficiency (RME) provides a way to assess how much reactant material ends up in a desired product at the end of a chemical reaction. One way of expressing RME is as follows:
![]()
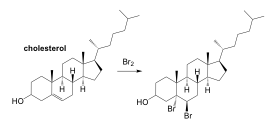
0.115 moles of cholesterol (C27H46O) reacted with 0.365 moles of molecular bromine to form 0.102 moles of dibromocholesterol in an addition reaction (shown below). It was possible to recover and recycle 0.151 moles of molecular bromine from the reaction mixture.
- 43.3%
- 54.3%
- 70.8%
- 84.6%
- 89.1%
Half of the students writing the exam got question number 17 correct, and the question had a discriminating index of 0.63. In this question, students were given a formula that might not be familiar to them and asked to interpret the correct use of that formula in the context of mol ® mass calculations.
The mass of cholesterol and bromine can easily be found with their given formulas. Students may have found it difficult to find the mass of dibromocholesterol because they needed to use the chemical equation.
Answer: 70.8%
- Vitamin-B3, or niacin, is an essential nutrient for humans. Its molecular structure is shown on the right.
A 0.0050 mol L–1 solution of niacin in water has a pH of 3.56. What is the percentage i onization of niacin in water?- 1.4%
- 2.8%
- 3.6%
- 5.5%
- 7.8%
Question 20 had a discriminating index of 0.60 and 49% of students answered the question correctly. This question involved a pH and % ionization of an acid calculation.
Please encourage your top students to take the exam. Students can win prizes worth $400 – 850 for the best results in your province and nationally. The 2017 CCC contest takes place on Monday, April 10.
References
- M. Amo-Salas, M. Arroyo-Jimenez, D. Bustos-Escribano, E. Fairén-Jiménez and J. López-Fidalgo, 2014, “New Indices for Refining Multiple Choice Questions”, Journal of Probability and Statistics, retrieved from www.hindawi.com/journals/jps/2014/240263/
- C. Rao, K. Prasad, S. Harish Permi, & J. Shetty, Item analysis of multiple choice questions: Assessing an assessment tool in medical students. International Journal of Educational and Psychological Researches, 2016, 201-204.
Acknowledgements
A team of chemistry educators from across Canada collaborate in the creation of the 25 multiple choice questions for Part A of the CCC. The 2016 CCC contributors were Dr. Sean Clapham,
Dr. Kathy Darvesh, Dr. Andy Dicks, Ken Hoffman, Danny Hickie, Jennifer Howell, Suzanne Monir, Jenny Pitt-Lainsbury and Mengting Qiu. Mengting also collaborated with me on the creation of the four topics for Part B of the 2016 CCC exam. The creation of original exam questions takes a great deal of time. The volunteer contributions of the writing team to providing top Canadian Chemistry students the opportunity to compete for monetary prizes is gratefully acknowledged, as is the time provided by the District and Regional Coordinators in marking the exams and the Donors for providing the monetary prizes. n