Scheduling an MRI (Magnetic Resonance Imaging) Scan.
Scheduler: No food or drink for four hours prior to the appointment time. You will be receiving an intravenous contrast agent. A recent blood test for creatinine level will be required.
Patient: What is the contrast agent?
Scheduler: Gadolinium.
Why is the heavy metal element gadolinium utilized in MRI scanning? Is there any chemistry involved? Yes. Read on.
Physics. MRI scanning1 is an application of proton nuclear magnetic resonance (NMR) to medical imaging. In MRI, the protons scanned are those of the water molecules in the scanned region. One of the many factors that determine the quality of the scan image is the rate at which excited protons in the scanned area relax, emitting radio frequency photons as they drop from the upper to the lower energy state in the magnetic field. A shorter relaxation time gives a better image quality.
Gadolinium1 is a lanthanide metal whose atoms and ions are highly paramagnetic.1 Gadolinium ions are introduced to the scanned region via an intravenous injection. These ions act to diminish the relaxation time of the protons, thus improving the contrast (quality) of the scan. This part of the story is the physics of scanning technology.
Chemistry. Gadolinium ions are toxic to mammals.1 Toxicity may be reduced by chelating the gadolinium ions with a multidentate ligand. A range of chemically tailored ligands has been synthesized for this purpose. All of these ligands form highly stable chelated complexes with gadolinium(III) ions.
Some of the complexes are general purpose. Others are more specialized, being preferentially absorbed into certain body fluids, cells or organs. For example, there is a gadolinium complex specifically for MRI scans of the liver and bile duct, and there are others which cross the blood-brain barrier to the central nervous system (CNS). This part of the story is the chemistry of gadolinium complex formation and the chemistry of the organic synthesis of specialized ligands.
Gadolinium in MRI contrast agents
Gadolinium. Gadolinium, symbol Gd, is element 64 in the periodic table. It is a lanthanide or rare earth element.1,2,3 There are six stable isotopes and numerous unstable isotopes formed by nuclear fission or other means. Gadolinium is estimated to be present at 6.2 ppm in the Earth’s crust. There are no major uses, but many special uses, due to its unique chemical and physical properties.
The electronic configuration of the Gd atom is [Xe]4f75d16s2. The electronic configuration of the Gd3+ ion, the most common chemical state, is [Xe]4f7. The half-filled 4f7-subshell is particularly stable, and has the maximum of 7 unpaired electron spins. The Gd atom and the Gd3+ ion are highly paramagnetic. The metal itself is ferromagnetic,1 as are many of its alloys. Some of the uses of gadolinium are: in magnet alloys, in alloys with iron and chromium that resist oxidation at high temperature and in phosphors.1
Gadolinium chelates. The Gd3+ ion is quite large as are many other lanthanide ions, and it has many available orbitals. It is commonly found to adopt coordination numbers up to nine and above. A number of chelating agents are used in commercial gadolinium MRI contrast agents,1 either off-the-shelf or chemically modified for a specific purpose. The following two examples illustrate this.
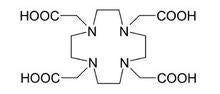
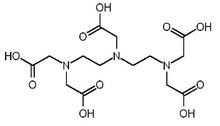
Among the modifiable off-the-shelf ligands utilized are DOTA1 (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) and DTPA1 diethylenetriaminepentacetic acid). These two octadentate ligands are super-sized versions of EDTA1 (ethylenediaminetetracetic acid). DOTA and DTPA are approved for medical use4 as ligands for the elimination of radioactive americium, plutonium, californium, curium and berkelium from the human body in cases of accidental exposure.
|
DOTA |
DPTA |
|---|
Fully coordinated, DOTA carries a charge of minus 4; fully coordinated, DTPA carries a charge of minus 5. DOTA is referred to in MRI terminology as a macrocyclic complexing agent, and DTPA as a linear complexing agent. The macrocyclic complexing agents tend to release gadolinium ions into solution more slowly than the linear complexing agents.
Gadolinium MRI contrast agents.1 Wikipedia lists nine gadolinium complexes allowed as gadolinium-based contrast agents (GBCA) for MRI. Some are ionic, some neutral. Of the nine GBCA, four including Gadovist®5 and Primovist®5 are listed in the 2011 Canadian Compendium of Pharmaceutical and Specialties1 (CPS).
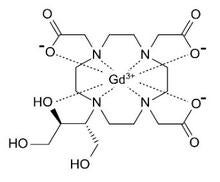
The chelated complex Gadovist whose generic name is gadobutrol is shown below left. Its chelating agent is a modified DOTA in which one acetic acid residue is replaced by a trihydroxybutyl (1,3,4-trihydroxybutan-2-yl) substituent. The fully coordinated ligand is octadentate and bears a minus 3 charge. The complex itself is neutral but is none-the-less extremely soluble in water. Gadovist is a general-purpose GBCA supplied as a 1.0 molar solution (604 mg/mL), allowing a relatively high concentration of gadolinium to be administered in a small volume (standard dosage = 0.1 mL/kg body mass).
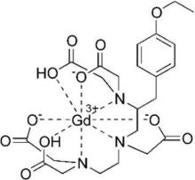
The chelated complex Primovist whose generic name is gadoxetate disodium is shown below right. Its chelating agent is a modified DTPA in which a substituent ethoxybenzyl chain replaces a hydrogen atom. The graphic shows the complex as being neutral, but the predominant form in body fluids at pH 7.4 is gadoxetate ion, in which all of the acetic acid groups are ionized. The chelating agent is octadentate and the complex ion itself bears a minus 2 charge. Primovist is a GBCA that is specific for liver and bile duct imaging.
|
Gadobutrol |
Gadoxetic acid |
|---|
Salts of unchelated gadolinium(III) are toxic, 1 but chelated gadolinium(III) is less so. However, chelated GBCA are by no means without potential adverse effects, 6 some serious, as evidenced in the product monograph of Gadovist. 7 Excretion is through the kidneys. Kidney health of the patient is indicated by blood creatinine 1 level, hence the patient’s requirement for a test result. The product monograph of Primovist, not available online, is here quoted from the CPS. Parenthetical comments have been added for clarity:
“…supplied as 181.3 mg/mL = 0.25 mmol/mL...
...dosage = 0.1 mL/kg body mass…
…Gadoxetate disodium = Gd-EOB-DTPA…
…log KGdL = 23.46 at pH 7.4, 37 °C…
…Gd3+ release rate = 0.07 % per day to 1.1 % at 15 days…
…GdL is a highly water soluble, hydrophilic linear compound with a lipophilic moiety (substituent side-chain), the EOB-ethoxybenzyl group. It does not pass the intact blood-brain barrier. It exhibits a biphasic mode of action: initially,
distribution occurs into the extracellular space, followed by selective uptake by the hepatocytes (liver cells) and biliary (bile duct) excretion, due to the lipophilic moiety. It is excreted approximately 50-50 by the renal route (kidneys) and the biliary route (liver). The excretion half-life in healthy 22-39 year old adults is 55 to 57 minutes, increasing slightly with age...”.
Questions for high school students
1. (a) Gadobutrol is a neutral complex, but it is extremely soluble in water. Why? (b) Gadoxetate disodium is an ionic complex that is water-soluble, yet is recognized as ‘fatty’ by the liver cells. Why?
2. Calculate the mg/kg of gadolinium in a dose of Gadovist for a patient. Compare this to the median lethal dose1 (formerly known as lethal dose 50% or LD50) as stated under Safety in the Wikipedia article “Gadolinium”.1 (15.7 vs 100 - 200 mg/kg)
3. The GBCA Primovist is supplied as a 0.25 M solution of gadoxetate ion. The product monograph gives the value of the equilibrium constant, called the formation constant or stability constant of the complex ion gadoxetate:
Gd3+ (aq) + L5- (aq) ⇌ GdL2- (aq) Keq = 2.9 × 1023
Reverse the equation.
(a) Calculate the value of the new equilibrium constant, called the dissociation constant or instability constant.
(b) Estimate the concentration of free Gd3+(aq) in the solution at equilibrium. (9.3 × 10-13 M)
(c) How may this concentration be measured by experiment?
References
- www.wikipedia.org for: MRI; gadolinium; paramagnetism; ferromagnetism; MRI contrast agents; DOTA; DTPA; EDTA; Canadian Compendium of Pharmaceutical and Specialties; creatinine; median lethal dose.
- Handbook of Chemistry and Physics, CRC Press, 54th Edition, 1973, page B14.
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, 2nd Ed., Butterworth Heinemann, 1997, Chapter 30, pages 1227-1249.
- CDC Fact Sheet, Radiation Emergencies: www.ametsoc.org/stationscientist/documents/dtpa.pdf.
- Compendium of Pharmaceuticals and Specialties, Canadian Pharmacists Association, 2011 print edition:Gadovist, page 1057; Primovist, page 1952.
- Nature Nephrology, What nephrologists need to know about gadolinium:
www.nature.com/nrneph/journal/v3/n12/full/ncpneph0660.html7. - Health Canada: search for Gadovist or DIN 02241089: http://webprod5.hc-sc.gc.ca/dpd-bdpp/newSearch-nouvelleRecherche.do?lang=eng.