Contact
 Juewen Liu, Department of Chemistry
Juewen Liu, Department of Chemistry
Introduction
Long-term exposure to arsenic threatens the health of over 150 million people across the world. Groundwater with naturally occurring arsenic at high levels is found in many countries, most notably in Bangladesh where over 30 million people rely on groundwater for drinking water, irrigation and other uses. Human activities, such as agriculture, mining and electricity generation, are also a source of arsenic contamination of surface and groundwaters. While very importance to measure and safeguard water and food safety, the detection of arsenic is a long-standing challenge in environmental analytical chemistry.
Traditional instrumentation methods for arsenic detection include atomic emission spectroscopy, atomic absorption spectroscopy, and inductively coupled plasma mass spectrometry. More recently, researchers have used biomolecules and nanomaterials for developing arsenic sensors. The purpose of the study was to critically review the emerging use of bio/nano sensors for arsenic detection based on the fundamental chemical interactions, and potential artifacts, between arsenic and related sensing molecules and materials with results being an important input for the further development and optimization of related arsenic sensors.
Methodology
The study reviewed the literature and fundamental chemical understanding to critically assess various methods using biomolecules and nanomaterials for sensing arsenic. More specifically, the study assessed and compared detection methods and sensors based on metal nanoparticles, metal nanoparticles coated in thiol, metal oxide nanoparticles and DNA aptamers (Figure 1). The detection methods reviewed included surface-enhanced Raman spectroscopy (SERS), fluorescence, colorimetry, and electrochemistry.
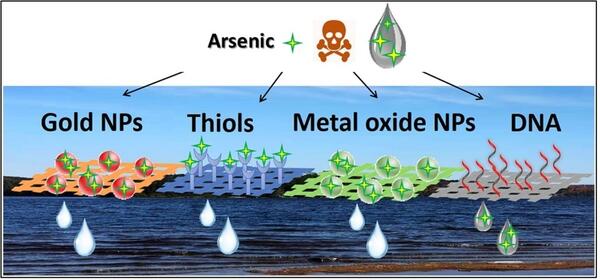
Figure 1: Arsenic binding interactions useful for sensor development.
Outcomes
Many arsenic sensors rely on metal nanoparticles and metal surfaces that can directly adsorb arsenic species, leading to various spectroscopic and electrochemical signatures useful for detection. Species include arsenite (As(III)) and arsenate (As(V)) which can inter-convert depending on the redox environment. Due to their chemical difference, the detection of arsenite and arsenate are often performed using different methods. For example, noble metal nanoparticles have a strong affinity to arsenite, while metal oxides tend to adsorb arsenate more strongly. Such selective adsorption forms the basis for many nanomaterial-based sensors. SERS and electrochemistry were identified as attractive methods for arsenic sensors due to their high sensitivity.
Taking advantage of the unique size, shape and environment-dependent properties of noble metal nanoparticles, colourimetric detection of arsenic has also been extensively reported. The review identified that disperse gold nanoparticles have an intense red colour which changes to purple or blue upon aggregation, a very attractive property for designing colorimetric sensors.
The review included several sensors based on methods that bind metal nanoparticles coated with a thiol ligand with arsenite. Published studies showed that thiol-containing molecules attached to gold nanoparticles where the presence of arsenite would aggregate and cause a colour change from red to blue. The review found that the interaction between arsenite and gold surfaces needs to be further considered in these systems as the colour change could also be produced by the displacement of the thiolated ligand by arsenite.
The interactions of arsenic species with metal oxides dominate the mobility and removal of arsenic in the natural environment. Metal oxides such as titanium oxide, iron oxide, manganese oxide, and cerium oxide are useful adsorbents because of their excellent arsenic removal efficiencies and provide new avenues for arsenic sensing. The review found that the fluorescence quenching property of these metal oxide nanoparticles allows a fluorophore-labeled DNA oligonucleotide to be adsorbed resulting in quenched fluorescence. Arsenate may displace the DNA to produce enhanced fluorescence for the detection.
DNA aptamers are single-stranded DNA oligonucleotides that can selectively bind to target molecules. Aptamers are therefore highly attractive for biosensor design due to their high stability and programmable structures. In 2009, a DNA aptamer was selected to bind to arsenic and has since gained popularity in biosensor development. The review found, however, that this aptamer sequence is unable to bind arsenite or arsenate and postulates that the previously reported success might be related to the binding assays which often contained gold nanoparticles or a gold surface. Similar to the metal nanoparticle/thiol ligan systems, the adsorption of arsenite to gold may have contributed to the observed signal. Since DNA is highly negatively charged and both arsenite and arsenate are anions, whether a high affinity aptamer sequence exists for them requires further investigation.
Conclusions
Bio- and nano-based systems and sensors remain promising for the detection of arsenic but require further research and development. Fundamental studies are needed to determine more specific arsenic binding materials and aptamers. In addition, more careful design, testing and evaluation of sensors, together with appropriate control experiments, are needed. For example, most current sensors were only tested against spiked water samples and require stringent testing against real environmental water samples, including a side-by-side comparison with standard instrumentation methods. For promising methods, converting systems from lab-based analysis to field deployable sensors is a critical next step requiring collaboration between chemists, engineers and environmental scientists.
Zong, C., Jin, X., Liu, J. (2021). Critical review of bio/nano sensors for arsenic detection. Trends in Environmental Analytical Chemistry, Volume 32, December 2021. https://doi.org/10.1016/j.teac.2021.e00143
For more information about WaterResearch, contact Julie Grant.






