The Arctic atmosphere is different. There are specific pollutant problems.1 There are also unique types of clouds: nacreous and noctilucent. But it is the awe-inspiring aurora that are the most well-known phenomena. For Inuit, the aurora light up the darkness in the long winter nights, and have a profound spiritual role in their culture. To chemists, the chemistry underlying them is of equal fascination and even today, there are still gaps in our knowledge about them.
Atsanik – The Northern Lights
As the reader would know from our previous articles, I (Chaim) am from a small, isolated Inuit community. There are no unfamiliar faces except for the few outsiders who fly in from the south. There are no roads beyond the perimeter of the town. So you can imagine as a little kid (of course, that was when I was mature enough to understand where I was, and how to get home) I had freedom to roam and explore what I thought was my big world as much as I pleased. I remember that little of my time was spent indoors, and anytime I was inside, it was because I was either sleeping or eating, or because my mom had told me to come in. This was the case for most of the children in our community.
 FIGURE 1
FIGURE 1
Aurora (Atsanik) over Arctic Bay, Nunavut, taken on January 3, 2022. Credit: Mark Long
For the protection of us fearless, exploring children, our parents would use a method in which they would tell us stories and “legends” about some of the potential threats to our safety. This was done in such a way that it was playful and we enjoyed it, while at the same time making us think very critically about our actions and the possible consequences of those actions. There are various versions of each story told, but all have similar resulting messages. Such parental monologues could range from short stories to – what children would perceive to be – a three-hour lecture. One of the stories told was that of the atsanik (northern lights), a story passed down through many, many generations of Inuit parents, and probably for thousands of years. The story is meant to keep children from staying out too late during the evening/night – a time that provides great opportunity for risks, and a time that parents prefer their little ones to be inside.
I was told (in parts) by my grandparents, my older siblings, and my friends, that the atsanik were a sign of our ancestors coming out to visit, to play, and to enjoy dancing in the night sky while everyone slept. Also, I was told, if you are whistling and playing in the night while they are out, they will become more colourful, more vibrant, and they will dance harder. As a child, this story was a great attention-grabber. I was very excited about the atsanik, and the next night I was going to stay up past my bedtime to go and play with the ancestors in the sky. However – and this is the part of the story that made me think very hard about what I was going to do – it was made clear to me that the brighter the lights got, the more they danced around, and the more they would have fun. There was a greater chance of them reaching down, chopping my head off, and using it to play soccer. A very effective method of making sure I was home safe every night! Variations of this story are told to children in every Inuit community from Greenland to Alaska – and probably across northern Russia.2 The legend has been turned into a beautiful story-book for Inuit children (Fig. 2).3
Science of belief
 FIGURE 2
FIGURE 2
Cover of an Inuit children’s book on the atsanik legend.
Growing into my adolescence and early adult years, the belief that the atsanik would use my head as a soccer ball dissolved, as did my childhood, and as would any other stories I was told as a child. However, the belief that the atsanik are our ancestors, dancing in the sky, coming to visit us, is still a part of my life. I believe the atsanik have the ability to make us Inuit feel very peaceful, comforted, and connected to each other as well as to the environment, feelings that simply cannot be explained by science: a belief.
Following from that, I want to express how interesting it has been to discover a world of core science, specifically chemistry, and to learn about the science underlying my beliefs. Throughout elementary grades, all the way up to high school, I was taught that the northern lights were waves of solar energy hitting the magnetic field produced by Earth’s gravitational pull. However, it was not until university studies that I learned the chemistry causing the light emissions (of which you will read below). So, being from a place of belief and little knowledge on the concept, and coming to a setting surrounded by scientific information on the northern lights, it has been an intriguing intellectual journey. Another great attention-grabber; but in this case, for chemistry.
The atmospheric layers
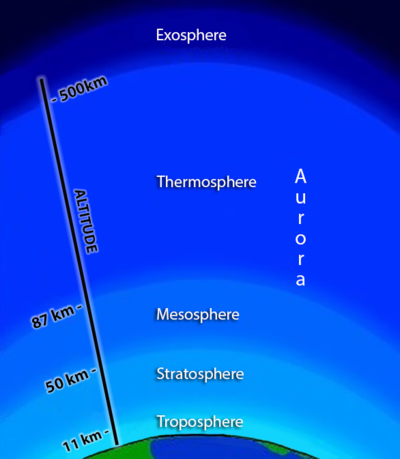 FIGURE 3
FIGURE 3
Schematic of the layers of the atmosphere. Adapted from: https://sites.google.com/site/layersofearthsatmosphere
Before we can make sense of the Arctic atmospheric phenomena, we must briefly review the structure of the Earth’s atmosphere.4 Though it looks as if the aurora are just overhead, they are, in fact, at the edge of space: above our own troposphere; above the stratosphere; and even above the mesosphere. They occur in the lower thermosphere, where the atmospheric pressure is incredibly low. These layers are shown diagramatically in Fig. 3.
Aurora borealis
Almost everyone has heard of the aurora borealis,4 the northern lights, or their southern counterpart, aurora australis. However, only those privileged to live in polar regions, like Chaim, really appreciate their beauty. What causes an aurora? As a result of motions within the Earth’s liquid metallic core, our planet has a magnetic field. The Sun expels streams of fast-moving sub- atomic charged particles, mainly electrons and protons, which we call the solar wind. The Earth’s magnetic field, known as the magnetosphere, deflects most of the particles as they approach the Earth; however, some travel down the polar ring of lines of force (Fig. 4). It is the collisions between these charged particles and the molecules of our upper atmosphere that result in the aurora.
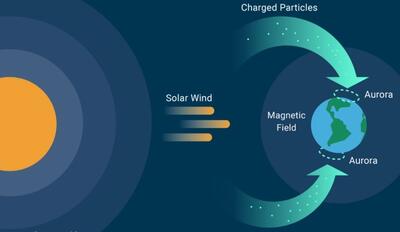 FIGURE 4
FIGURE 4
A schematic of the solar wind causing the aurora. Adapted from: https://www.timeanddate.com/astronomy/northern-southern-lights.html
The aurora occur mostly between altitudes of 90 km and 150 km, though they can occur at altitudes of up to 1,000 km.5 The aurora borealis are most intense between latitudes of 10° and 20° from the geomagnetic poles, but during solar storms they can be observed in much more southern locations. In the thermosphere, the atmosphere is so thin that seconds, or even minutes, can elapse between collisions of molecules, atoms, or ions. As a result, exotic species exist for measurable times, and it is reactions involving these that give rise to the aurora.
The most common colour of aurora is green, and the emission is known as the auroral green line. The origin of this emission is as follows. At an altitude of about 90 km to 100 km, dinitrogen molecules are common. A solar-wind particle can impact a dinitrogen molecule, causing an electron in the molecule to be raised to a higher (excited) energy state, which we depict by an asterisk:
N2 + energetic particle —› N2* + particle
At this altitude, the dioxygen covalent bond is weaker than the dinitrogen bond (498 vs. 946 kJ·mol-1). Therefore, as a result of the high energy impacts occurring, most dioxygen atoms are broken apart into free oxygen atoms. These free oxygen atoms are then able to react with the excited N2* particles. A collision between an excited dinitrogen molecule and an oxygen atom excites an electron in the oxygen atom to a much higher energy state:
N2* + O —› N2 + O**
This energy state only has a lifetime of 0.7 seconds. Since this high energy state cannot be sustained, the electron falls back down to a lower energy level, emitting energy to do so. This emitted energy is in the form of an electromagnetic wave, with a wavelength corresponding to the green part of the visible spectrum:
O** —› O* + green photon
The lower-excited energy state oxygen atom has a lifetime of 110 seconds and usually in the intervening time the atom has collided with some other atom or molecule and lost the energy, returning to the ground state of the oxygen atom.
The second most common colour of aurora is red. The auroral red line emission occurs at a higher altitude, typically about 150 km. Up at this altitude, there are free electrons from the solar wind, and the collisions between atoms are so infrequent that excited oxygen atoms have time to lose their energy by photon emission:
O + e-* —› O* + e-
O* —› O + red photon
Then higher still, there is the blue emission. At this altitude, bombarding electrons can ionize dinitrogen molecules (1,503 kJ·mol-1), leaving a molecular cation with an electron in an excited state. As the electron drops to the ground state, it emits a photon in the blue part of the spectrum.
N2 + e-* —› N2+* + 2e-
N2+* —› N2+ + blue photon
Fig. 5 shows an auroral display where both blue and green are visible.
 FIGURE 5
FIGURE 5
Aurora, showing the blue and green layers, at sea off Kitlineq (Victoria Island), an island shared between Inuvialuit and Nunavut. Credit: Getty Images
Commentary
Observations of the polar atmosphere provide a special link between Inuit and scientists everywhere. We are all in awe of these amazing natural phenomena. And it is through atmospheric chemistry that we can understand their causes. Yet to Inuit, they are also a spiritual link. Heading back north after time in the south, the sight of the atsanik reunites Chaim, and all Inuit, with their people and their homeland.
References
- Andersen, C.C.; Rayner-Canham, G. PFOS: the newest Arctic Pollutant. Chem 13 News, November 2018.
- Davis, T.N. The Aurora Watcher’s Handbook; University of Alaska Press, 1992, pp. 166-167; Falck-Ytter, H. Aurora: The Northern Lights in Mythology, History and Science; Bell Pond Books, 1999, pp. 29-44.
- Kusugak, M. Northern Lights: The Soccer Trails; Annik Press, 1993.
- Wikipedia. Atmosphere of Earth. https://en.wikipedia.org/wiki/ Atmosphere_of_Earth (accessed 2019-06).
- Wikipedia. Aurora. https://en.wikipedia.org/wiki/Aurora (accessed 2019-06).
Publisher's note: This article is a reprint from the September 2019 issue of Chem 13 News.







