I have used this project to give the students an opportunity to apply a simple art form to their studies of chemical kinetics in grade 12 chemistry. In the unit the students study reactions and mechanisms, where concepts such as bond breaking/making and the activated complex are discussed. Many students don’t clearly understand the changes in molecules that occur when reactions take place. The formation of the activated complex as a structure with definite bond angles and bond lengths in the process of changing is hard for some to visualize.
First the student chooses a simple chemical reaction with compounds that are easily drawn in the molecular category. The teacher should check this choice. Ionic compounds don’t lend themselves to this activity. Covalently bonded 3 – 10 atom molecules of carbon, nitrogen, sulphur, phosphorus, hydrogen, oxygen and the halogens are preferred. The balanced equation is written and is preferred with a low order. This prevents too much clutter in the finished product.
The idea is to construct a flip chart animation that will show the approaching reactant molecules, the formation of the activated complex and the final product formation.
Equipment
- pad 12 cm x 12 cm x 4 cm (min) thick would be adequate
- packing or duct tape to reinforce the glued section of the pad
- coloured pens for atoms other than carbon
- black pen for carbon and the bonds
Process
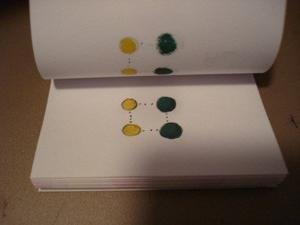
- The student begins by drawing the reactants and products in 3D structural formulae considering real bond angles and bond lengths that can be properly represented on a 2D surface. Atoms can be colour-coded, and the bonds are solid lines in black.
- The proposed activated complex can also be drawn showing the bonds changing (dotted lines) and the bonds that remain intact (solid).
- The size of the molecules and layout becomes the next challenge in placing the molecules on the pad. Also, the visibility of the reaction as you flip the pages should be considered.
- The molecules are laid out carefully on each successive page of the pad starting from the back. Remember you want the images to flow as smoothly as possible from each frame.
- Energy factors such as activation energy and the exothermic or endothermic energies can be added with flashes of lightning or such.
- When the students have completed their animation they can package it however they like. Some have submitted their product in a box or labelled, hand-made container. A colour key should be included along with the balanced equation and some information about the reaction — uses, conditions, state etc.
The concept can also be used to show heterogenous/homogenous catalytic mechanisms with the interactions between the catalyst and the reactants.
I think it is very important that we try to include other disciplines in our subject. This also provides us with another activity in “differentiated instruction”.
“Snap-shots” of a sample flipchart are pictured to the right.