Having taught chemistry at all levels for many years, I know that teachers are all about borrowing/stealing from other teachers — God knows I did my share as a beginning teacher. Even with the wealth of resources out there, though, sometimes I was unable to find handouts with the content I wanted. I also noticed that students tended to lose their handouts, so I set about solving these two problems by making my own handouts that were so fun that students would want to save them all — and hopefully actually read them. In an effort to give back to the community that has helped me so much, I've starting posting handouts on my Cation Design blogspot.
Shown below and on the next page are a couple of my handouts. I’ve been surprised by how much of a difference some cute pictures can make; you’d think that high school students were above that sort of thing, but apparently not! I’ve had alumni come back and tell me that these handouts were the only notes they kept from high school, and that they found them helpful even in their general chemistry classes in college.
The blog has handouts on making observations, evidence of chemical reaction/change, solutions and the electromotive force series. As well, there are a couple of mole cartoons.
One of the constant challenges we face as chemistry teachers is lab safety. It can be easy for students to become blasé about that beginning of the year lecture, especially if they feel like they hear it every year, so I switch things up with the following handout. Instead of me telling them what not to do, I ask students to pick out what’s wrong. From the silly (icing a cake with a pipet!) to the serious (wafting instead of inhaling fumes directly), there are enough things to point out that a good number of students can be given a chance to participate. This drawing was actually done by my sister; she was a chemistry student herself when I first started teaching, and I commissioned her to do this piece to help me out.

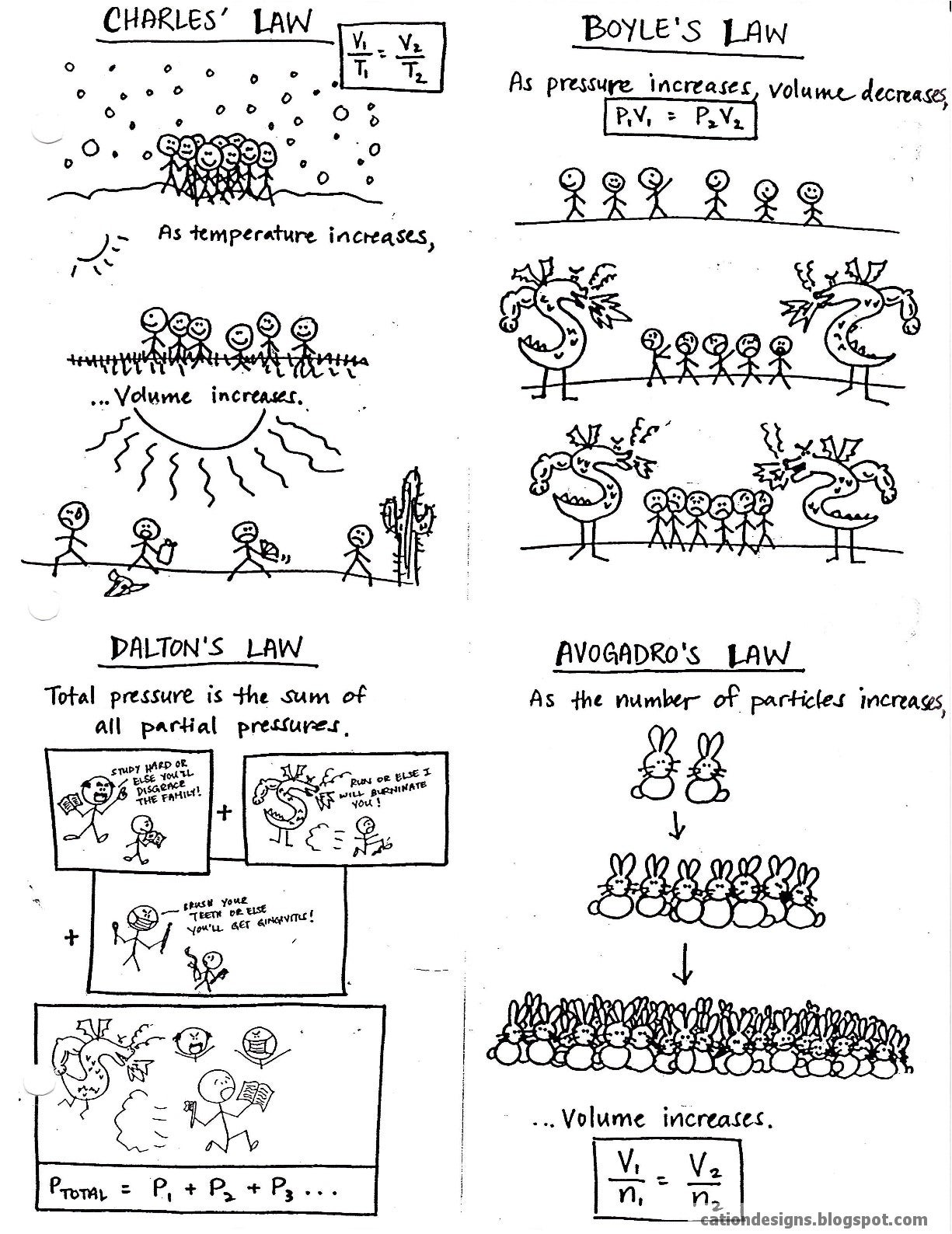
This gas laws handout summarizes the main laws, as well as provides tongue-in-cheek illustrations of the concept. I introduce each law and use the comics to help students remember what the calculations mean in terms of particle behavior. For example, with Charles’ law, I use the analogy that when it’s cold outside, people are happy to huddle together, but once it gets warmer — and they get sweatier — they move farther and farther apart. For Boyle’s law, pressure in the form of a dragon causes the people to get closer together, i.e., decrease their volume. Dalton’s sum of partial pressures is like when students have multiple stressors, which are cumulative, resulting in the feeling of intense pressure or stress on them.







