Energetics for Photoionization
PhotoMateTM lamp
Krypton 10.0 eV and 10.6 eV
Ionization Potentials (IP)
Anthracene 7.4 eV*
Fluoranthene 7.8 eV*
Caffeine 8.0 eV*
4-Nitrotoluene 9.5 eV*
2,4,6-Trinitrotoluene 10.59 eV
Dopant Ionization Potentials
Toluene 8.82 eV*
Acetone 9.70 eV*
Solvent Ionization Potentials
Methanol 10.85 eV
Acetonitrile 12.19 eV
Water 12.61 eV
* Ionized by Kr UV photons
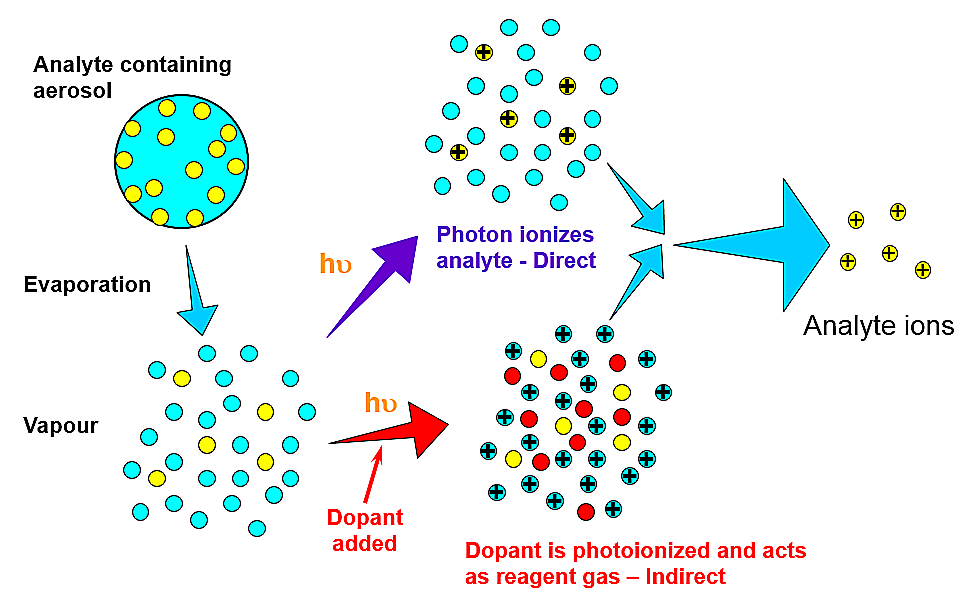
- The photons from the Kr lamp can only photoionize compounds of lower IP
- Common HPLC solvents (H2O, CH3OH and CH3CN) are NOT ionized and therefore cannot aid ion formation
-
In
this
circumstance,
only
direct
photoionization
of
the
analyte
can
yield
characteristic
ions
such
as
M+.
(not
very
efficient)
- subsequent ion/molecule reactions can form [M+H]+
- Dopants are used that will be ionized by the Kr lamp