A catalyzed decomposition reaction with explosive results!
How it works
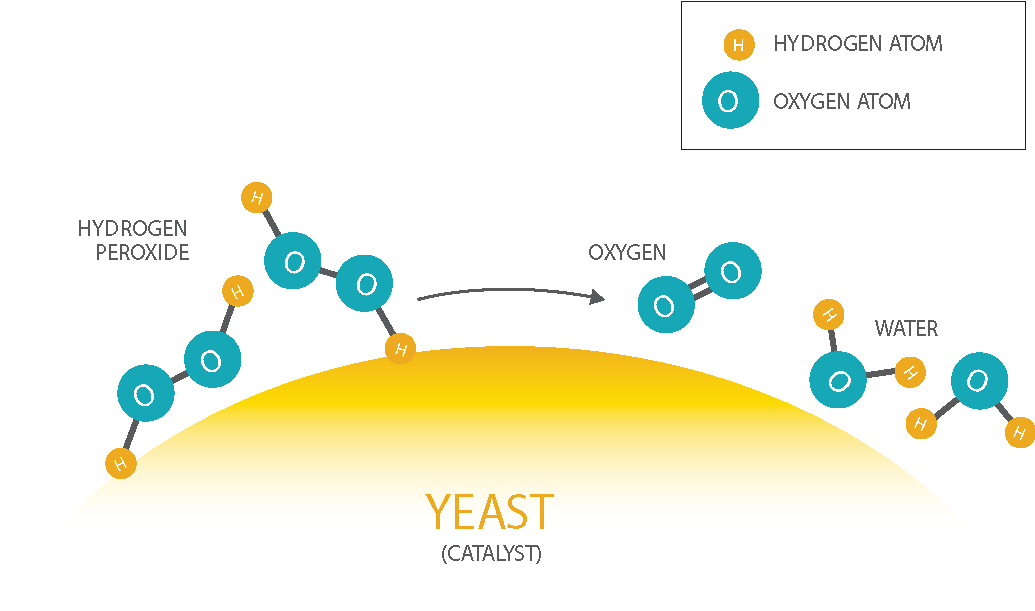
Hydrogen peroxide (H2O2)is a chemical that is made of two hydrogen and two oxygen atoms. Over time, it slowly decomposes into water (H2O) and oxygen gas (O2). Yeast acts as a catalyst to speed up the reaction, and the soap bubbles trap all of the rapidly produced oxygen.
Try this at home
Do you like exciting chemical reactions? Try mixing common household acids (vinegar, lemon juice, orange juice) with baking soda to see what happens!
Definitions/glossary
- Chemistry: a branch of science that examines properties and interactions of matter
- Catalyst: a substance that speeds up a chemical reaction without being affected
- Decomposition Reaction: a chemical reaction that involves a larger molecule breaking up (or decomposing) into smaller molecules
What's going on at the University
Velocity Science provides students interested in a life or materials science startup with the right tools and resources to initiate and develop world-class science companies. One of these companies is Suncayr.
Suncayr is making sun safety simplified with a colour changing marker to tell you exactly when your sunscreen is no longer protecting you. This is drawn directly on the skin before applying sunscreen, and when the sunscreen is no longer blocking UV rays, the ink changes colour.
How to become a chemist
The first step to becoming a chemistry student is to graduate from high school with the required math, science, and english courses. After high school, you will attend a post-secondary program. Chemistry students at Waterloo prepares are prepared for a growing number of dynamic career options. They could be searching for the roots of crippling diseases, developing cancer-fighting drugs, or assessing environmental damage.