We are happy to share our latest work published in ACS Electrochem: "Diffusion-Limited Stability of Lithium Metal Anodes in LiFSI/Glyme-Based Solvate Ionic Liquid Electrolytes." The paper was first authored by group member Ivan Kochetkov.
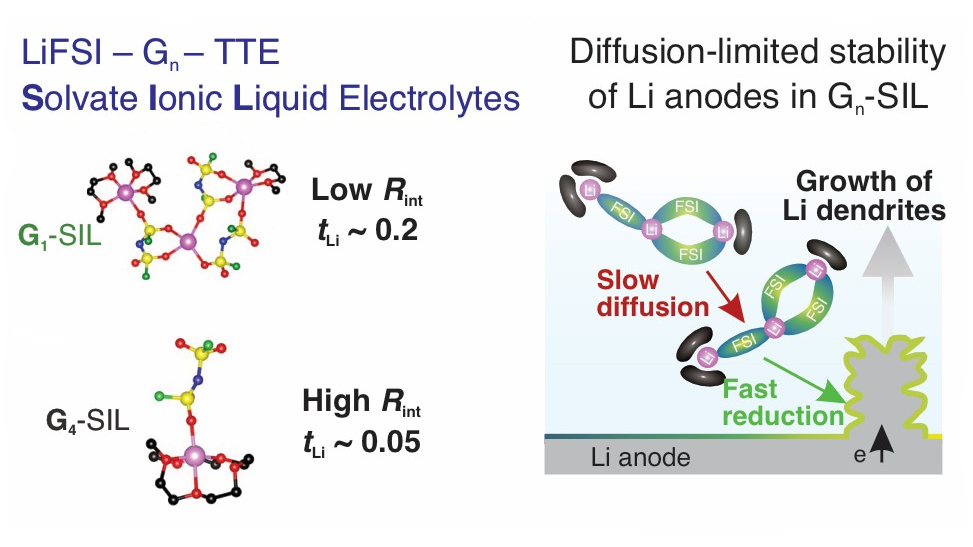
Localized high-concentration electrolytes containing bisfluorosulfonyl imide (LiFSI), dimethoxyethane, and fluoroethers greatly extend the lifetime of lithium metal batteries compared to conventional organic carbonate-based electrolytes. However, the intrinsic flammability of dimethoxyethane (G1) presents a safety concern, motivating the exploration of more thermally stable alternatives such as diglyme (G2), triglyme (G3), or tetraglyme (G4). This study investigates the effect of glyme length on Li+ transport and the stability of lithium metal anodes (LMAs) in a series of solvate ionic liquid (SIL) electrolytes containing LiFSI, Gn (n = 1–4) and 1,1,2,2-tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether in a 1:1:4 mole ratio. The combination of impedance spectroscopy, Raman spectroscopy, and DFT calculations reveals an approximately 10-fold increase in the interfacial impedance of LMAs (from 20 Ω·cm2 in G1 to 200 Ω·cm2 in G4) due to the chelation of Li+ ions by longer-chain glymes. A similar trend is observed in the lithium transference number, which decreases from 0.22 in G1 to below 0.05 in G3 and G4. As a result, the slow diffusivity of Li+ ions in SIL electrolytes limits the rate capability of LMAs in asymmetric Cu–Li cells and full cells with high-loading (19 mg·cm–2) LiNi0.8Co0.15Al0.5O2 positive electrodes to ∼1 mA·cm–2 at room temperature. Overall, this work presents a systematic framework underlying the transport limitation of glyme-based SIL electrolytes for their applications in LMBs.
DOI: 10.1021/acselectrochem.5c00395