Research areas
Our discovery research focuses on using hypervalent iodine (HVI) reagents in organic synthesis.
Our industrially-relevant research focuses on deuteration of small-molecule organics.
Check out our current projects below or on our Publications page.
Current projects
Iodonium ylides are similar to pre-formed metallocarbenes, easily undergoing cyclopropanation and X-H insertions. These reactions occur either in the presence of catalysts or under blue LED irradiation.
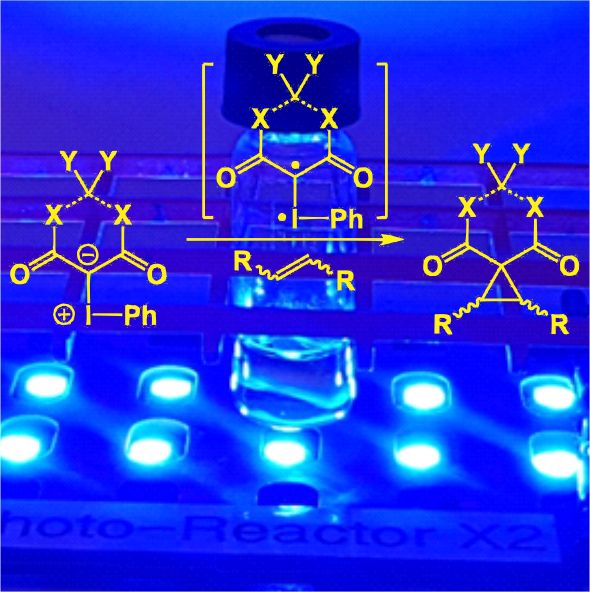
One emerging theory we are studying computationally and experimentally is the role played by an iodonium ylide's sigma holes in metal-free reactions.
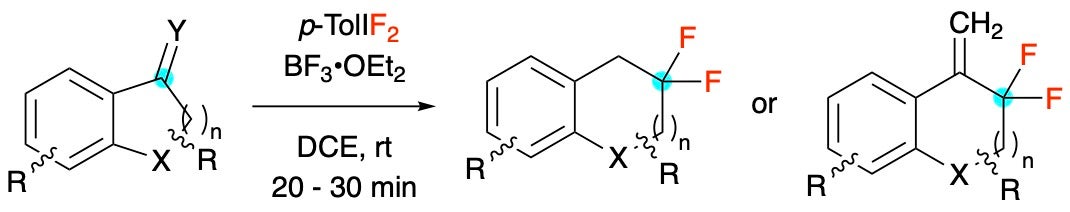
Fluorinative Rearrangements of alkenes and allenes
F-containing HVI reagents can transfer fluorine atoms to unsaturations like alkenes and allenes. When phenyl-substituted, 1,2-aryl migrations can occur to produce novel fluorinated motifs.

Incorporation of radioactive fluorine (18F) in biologically relevant molecules
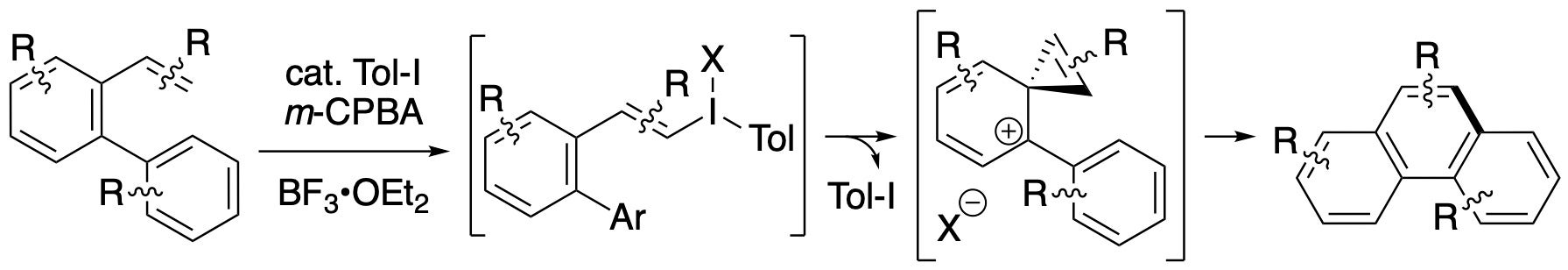
Iodoarene catalysis for C–C bond formation
Iodoarene catalysis was used to achieve an intramolecular coupling of arene and alkene C–H bonds, where the hypervalent iodine catalyst was produced in situ from iodotoluene and m-CPBA. This novel reaction appears to proceed though vinyliodonium and vinylenephenonium intermediates.
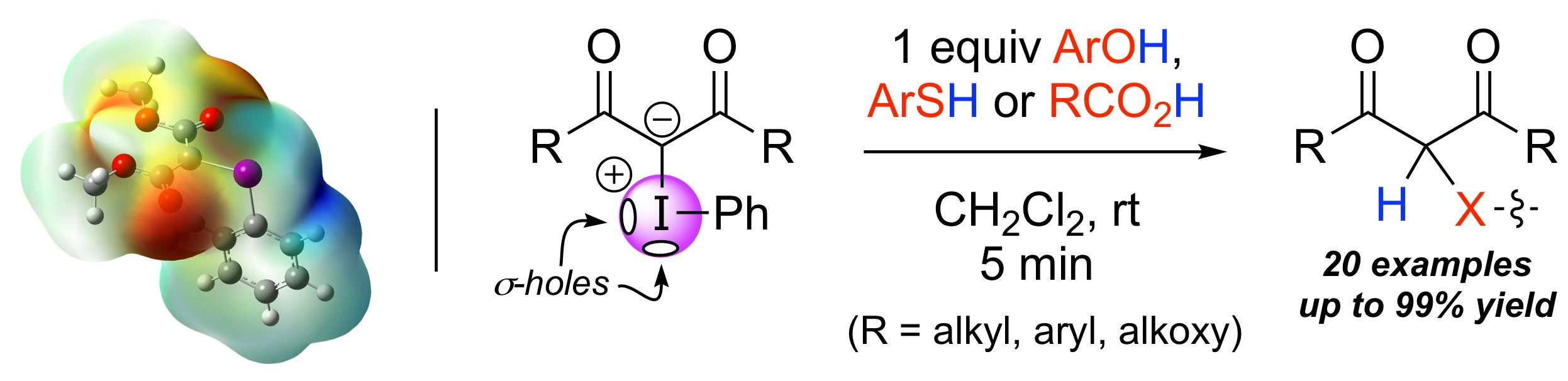
a-Carbonyl Functionalizations using HVI reagents
Functionalizing the a-carbonyl position with HVI reagents traditionally transfers either X1 or X2 to a substrate. If the substrate already possessed a suitable leaving group, both of the lignads can be attached. This is a highly effective way to do metal-free, double alpha-carbonyl halogenation in a single transformation.

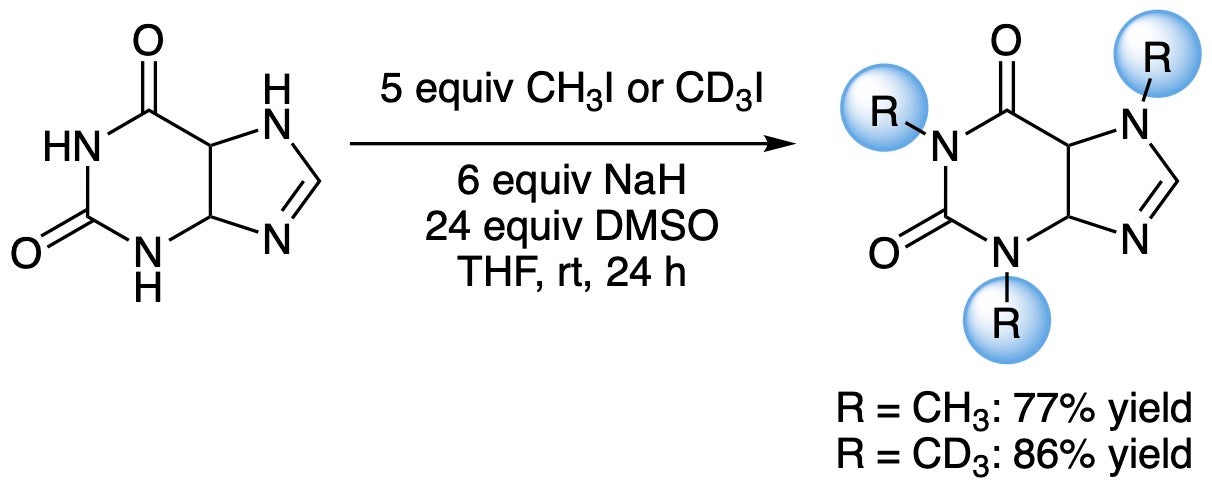
Deuteration of small-molecule organics (in collaboration with deutraMed Inc. of Collingwood, ON)
Deuteration of organic molecules is an important emerging strategy in organic materials and pharmaceutical sciences. Creating a value-addition ladder for deuterium oxide (D2O) and deuterium gas (D2) is the central theme of this research collaboration.
If you are interested in our research, or would like to learn more about what we do, feel free to contact Graham Murphy or stop by C2-367.