Nitrogenase: A Molecular Inorganic Perspective
The nitrogenase enzymes fix atmospheric dinitrogen through the action of a singular class of metallocluster cofactor. For the most studied, most active enzyme subclass, this cofactor has been formulated as a structurally complex [MoFe7S9Q] cluster (the iron-molybdenum cofactor, FeMo-cofactor), where Q is a recently discovered interstitial monoatomic ligand believed to be nitride.

To inorganic chemists, the FeMo-cofactor presents a number of longstanding challenges. Neither the synthesis of the cofactor nor its mechanism of operation is understood. The substrate binding site is unknown, the cluster charge state is uncertain, and the relationship of the cofactor structure, which derives from a resting state enzyme, to catalytically-active forms is unclear. In short, biological nitrogen fixation is very much a mystery at the molecular level.
In the Lee lab, we are investigating synthetic iron-nitrogen and iron-nitrogen-sulfur clusters to gain insight into the chemistry of the FeMo-cofactor. We adopt a broad, biologically-inspired approach that targets small molecule complexes which share critical properties (real or proposed) with the biological system. By defining the fundamental chemistry of congruent complexes, we hope to delineate chemical possiblities in the biological system. Some topics of interest arising from our studies follow.
Iron-Nitrogen Clusters
Current mechanistic conjectures suggest that dinitrogen activation occurs through bridging interactions with the central iron sites in the FeMo-cofactor, with the substrate integrated into the cluster framework as N2 or N fragments (at various redox and protonation states) during the reduction process. While the recent discovery of the putative nitride ligand within the cofactor is suggestive in this context, direct experimental support for this hypothesis is lacking. To better understand the interaction of iron and nitrogen in the context of nitrogenase chemistry, we are developing the largely unexplored chemistry of weak-field iron and nitrogen anion core ligands. We have made considerable progress in establishing synthetic access to these previously unknown species and characterizing their physical and chemical properties.

Sulfide/Nitrogen Anion Cluster Cores
The heterogeneous molybdenum/iron nitride/sulfide composition of the FeMo-cofactor core poses a challenging problem in inorganic synthesis. The synthesis of heterometallic iron-sulfide clusters has been extensively explored, and practical synthetic approaches exist. The systematic assembly of heteroligated cluster cores, however, is not well-developed, particularly as applied to weak-field metal systems. We are actively pursuing synthetic tactics to address this latter problem.
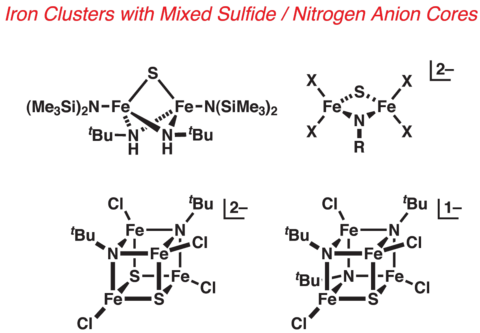
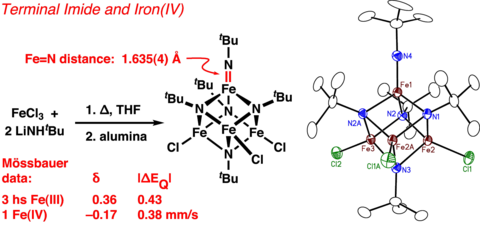
Novel Iron Environments
In our studies of iron-nitrogen cluster chemistry, we have discovered complexes containing both high-valent metal centers (+IV oxidation state) and stable terminal imide moeities. At iron, the high-valent +IV oxidation state is rare and the terminal imide group was unknown prior to our discovery; both are associated with reactive oxidation chemistry with implications in synthesis, catalysis, and bioinorganic mechanism. We are working to understand the extent of this motif and its potential for chemical reactivity.
Other Topics
In addition to our focus on nitrogenase-related chemistry, we also have interests in other areas of transition metal inorganic chemistry. Some examples are given below.
Divalent Titanium
Reduced early transition metal centers are of longstanding interest due to their high chemical reactivity, particularly with respect to normally "inert" substrates. In our lab, we have utilized the classic tris(pyrazolyl)borate (Tp-) ligand to introduce an isolable, yet reactive, divalent titanium center in the form of Tp2Ti. Our synthesis of Tp2Ti suggests the accessibility of a broad chemistry using polypyrazolylborates as versatile ancillary ligands for these metal sites.

Computational Inorganic Chemistry
Density functional theory (DFT) provides a powerful computational tool to augment experimental methods in inorganic chemistry. In collaboration with the research group of R. H. Holm (Harvard), we are exploring the use of DFT calculations to analyze thermochemical and stereochemical issues related to the synthetic analogue chemistry of molybdo- and tungstoenzymes.
