The isometric crystal system has a unit cell in the shape of a cube. It is one of the most common and simplest shapes found in crystals. The crystallographic axes used in this system are of equal length and are mutually perpendicular, occurring at right angles to one another.
All crystals of the isometric system possess four 3-fold axes of symmetry, each of which proceeds diagonally from corner to corner through the centre of the cubic unit cell. It is the most symmetrical system possible in three dimensional space.
The isometric crystal system has the following forms:
- Cube
- Octahedraon
- Dodecahedron
- Trapezohedron
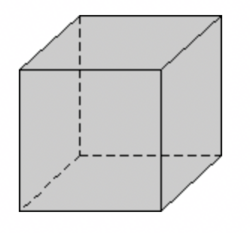
The cubic form consists of 6 square faces at 90 degrees to each other. Each face intersects one of the crystallographic axes and is parallel to the other two.
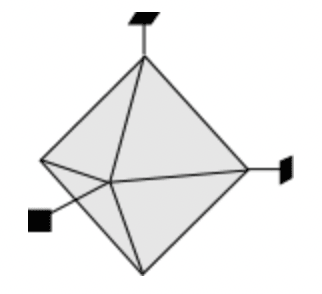
The octahedron form is composed of 8 equilateral triangles. The triangle-shape faces intersect all three crystallographic axes at the same distance.
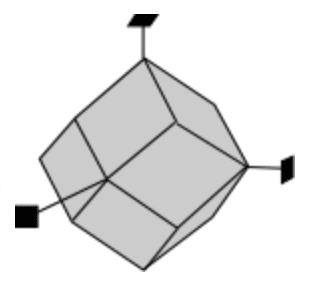
The dodecahedron form is composed of 12 rhomb-shaped faces. Each rhomb-shaped face intersects two of the other axes at equidistance and is parallel to the 3rd axis.
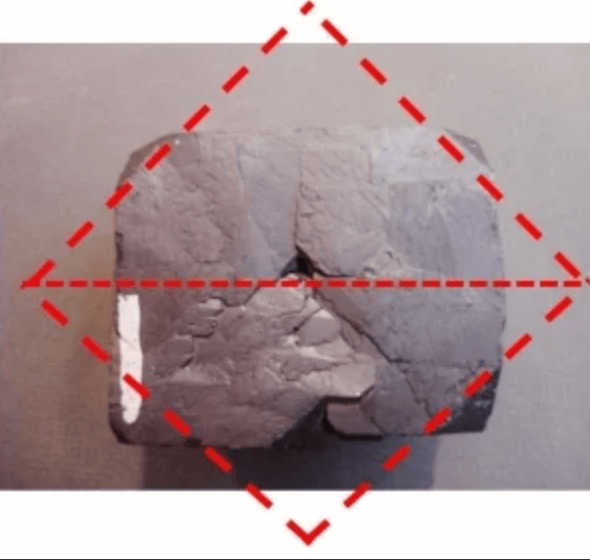
Galena, Isometric system cubic and octahedron form.

Galena, Isometric system cubic and octahedron form.

Alum (man made), Isometric System Octahedron Form.
Diagrams from What is Crystallography and Rockhounds.