Research and development
The innovation process generally begins with research and development of an innovation. The initial research should show evidence in support of the clinical effectiveness of the innovation.
Innovators in the research and development phase can facilitate a smoother transition through the innovation process by looking ahead and considering:
- Would this technology qualify as a medical device?
- Who is the target end-user?
- How will this device be marketed, and who will it be marketed to?
- How will end-users inform development of the technology?
- What reimbursement opportunities exist for this technology?
- What performance indicators have you chosen to provide rationale for the investment of dollars in this product?
- e.g. Has this product made a difference in the efficiency of care delivery, effectiveness of clinical outcomes for patients, reduction of length of stay in traditional institutions, and so on.
- Are you familiar with the content in current databases, such as interRAI, to be able to select appropriate outcome measures early on? You may find the following resources helpful:
Research may be necessary for obtaining regulatory licences and approval. See below for some licensing considerations you should make when developing your product.
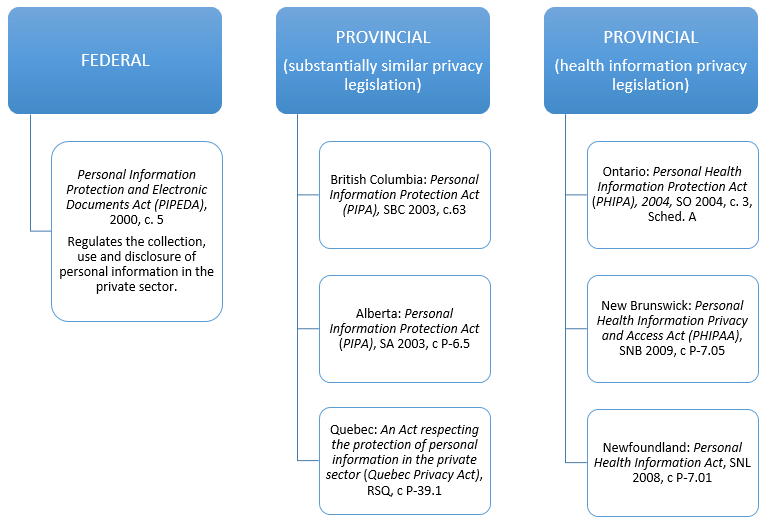
Privacy
Below is an overview of the key privacy legislation, noting that other legislation, regulatory guidance, and privacy best practices should also be consulted depending on your particular device. Bear in mind that devices that store a patient’s personal health data in order to better monitor, diagnose, or care for a patient fall under Canada’s privacy legislation.