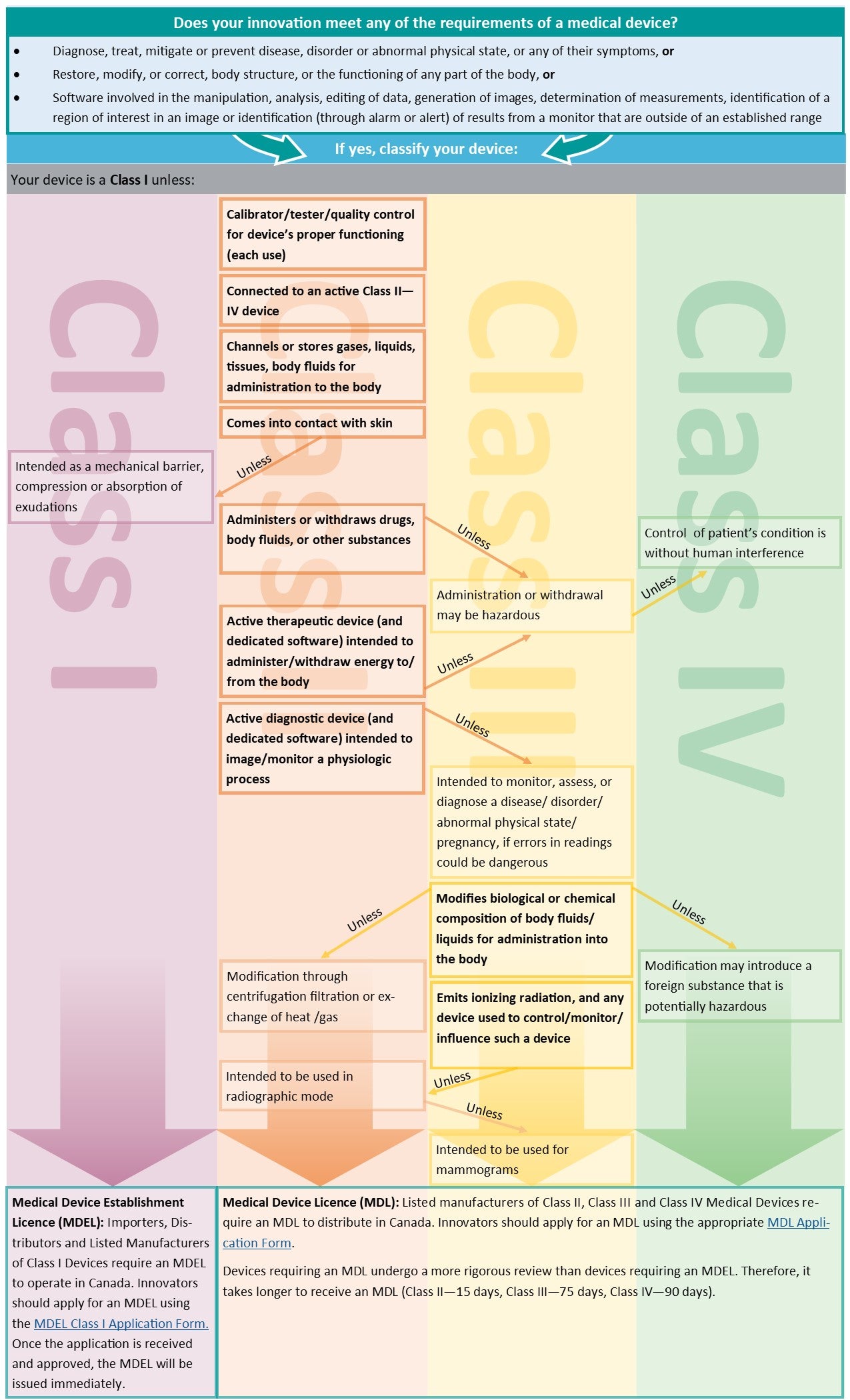
Licensing and regulatory approval
Does your innovation:
- Diagnose, treat, mitigate or prevent disease, disorder or abnormal physical state, or any of their symptoms
- Restore, modify, or correct, body structure, or the functioning of any part of the body
- Have software involved in the manipulation, analysis, editing of data, generation of images, determination of measurements, identification of a region of interest in an image or identification (through alarm or alert) of results from a monitor that are outside of an established range
Note: Consider how the device will be marketed or advertised. Making specific health claims (e.g. for a specific medical condition) may trigger a medical device classification.
If you have further questions about licensing and regulatory approval, contact Health Canada.