 Sex differences in blood pressure regulation
Sex differences in blood pressure regulation
Should men and women suffering from high blood pressure be prescribed the same medications?
Hypertension is a global health challenge with known sexual dimorphism in pathophysiology and in responses to drug treatments, yet men and women are typically treated with the same approach. We conduct model simulations to gain mechanistic insights into how sex differences in the renin-angiotensin system and in renal phenotype impact kidney function in hypertension.
 Simulating drug interactions in diabetes
Simulating drug interactions in diabetes
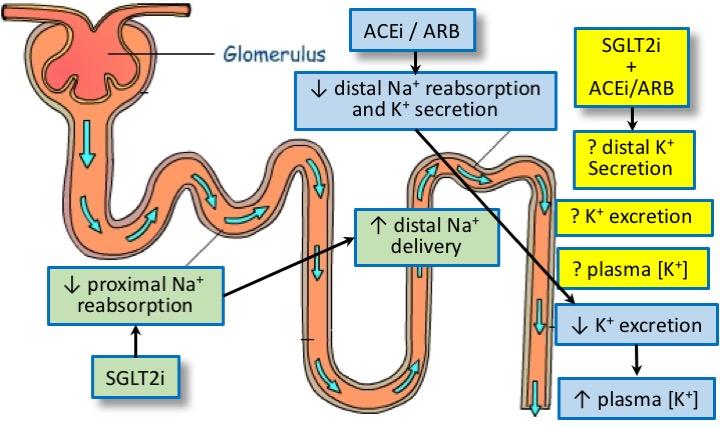
Described as an "economic tsunami" by Diabetes Canada, diabetes has been projected to incur an annual cost of $16 billion in Canada by 2020. We seek to understand the sex differences and drug-drug interactions of key anti-hypertensive treatment (ACE inhibitors and ARB) and anti-hyperglycemic treatment (specifically, SGLT2 inhibitors).
A known complication of ACEi and ARB is hyperkalemia (high plasma potassium concentration). The impact of SGLT2i on potassium balance is controversial. Only one of the SGLT2i (canagliflozin) but not the other three (dapagliflozin, empagliflozin, ertugliflozin) mentions an increased risk of hyperkalemia in its official monograph. To what extent does SGLT2i modify the risk of hyperkalemia in diabetic patients with preserved versus impaired kidney function? What are the potential drug interactions between SGLT2i and ACEi/ARB? And are there sex differences?
Exercise and intermittent fasting, for men versus women
 The increasing prevalence of obesity throughout the world is associated with an escalating incidence of obesity-related disorders and health costs. The increased health risks due to obesity vary depending on the location/accrual of adipose tissue. Specifically, adipose tissue distributed in the abdominal or visceral region carries a much greater risk for metabolic disorders, than does adipose tissue distributed subcutaneously. Differences in distribution of adipose tissue and the relative risk for diseases suggest that not all adipose tissue is created equally. There are distinct sex-dependent differences in the regional fat distribution. If age and body mass index (BMI) are matched, women have lower waist-to-hip ratio, indicating a greater amount of subcutaneous adipose tissue than men do. Excess adiposity in the central visceral region of the body (‘android’ or male-pattern obesity) is correlated with increased risk and mortality from disorders including diabetes, hyperlipidemia, hypertension, and atherosclerosis. In contrast, excess adiposity in the gluteal/femoral subcutaneous region (‘gynoid’ or female-pattern) is poorly correlated with risk for these metabolic disorders.
The increasing prevalence of obesity throughout the world is associated with an escalating incidence of obesity-related disorders and health costs. The increased health risks due to obesity vary depending on the location/accrual of adipose tissue. Specifically, adipose tissue distributed in the abdominal or visceral region carries a much greater risk for metabolic disorders, than does adipose tissue distributed subcutaneously. Differences in distribution of adipose tissue and the relative risk for diseases suggest that not all adipose tissue is created equally. There are distinct sex-dependent differences in the regional fat distribution. If age and body mass index (BMI) are matched, women have lower waist-to-hip ratio, indicating a greater amount of subcutaneous adipose tissue than men do. Excess adiposity in the central visceral region of the body (‘android’ or male-pattern obesity) is correlated with increased risk and mortality from disorders including diabetes, hyperlipidemia, hypertension, and atherosclerosis. In contrast, excess adiposity in the gluteal/femoral subcutaneous region (‘gynoid’ or female-pattern) is poorly correlated with risk for these metabolic disorders.
Exericse is a great way to lose weight or improve health. During aerobic exercise, women oxidize significantly more lipids and less carbohydrates than men. This sexual dimorphism in substrate metabolism has been attributed, in part, to the observed differences in epinephrine and glucagon levels between men and women during exercise. Others practice intermittent fasting, a diet schedule that cycles between not eating and eating. Cycles of intermittent fasting can be hourly or daily. How does the combination of exercise and intermittent fasting affect men and women differently? And how do the benefit differ between healthy and obesity individuals?
We have previously developed a mathematical model of the whole-body metabolism to predict fuel homeostasis during exercise by using hormonal control over cellular metabolic processes. The whole-body model is sex specific, and is composed of seven tissue compartments: brain, heart, liver, GI (gastrointestinal) tract, skeletal muscle, adipose tissue, and “other tissues”. Each tissue compartment is described by dynamic mass balances and major cellular metabolic reactions. The model is developed for a lean person and does not distinguish between visceral and subcutaneous fat. Here we will extend the model to (i) represent an obese person (both sexes) and (ii) incorporate the regional fat distribution. We conducted simulations to test the hypothesis that sex differences in the exercise-induced changes to epinephrine and glucagon would result in the sexual dimorphism of hepatic metabolic flux rates via the glucagon-to-insulin ratio (GIR). An ongoing project is to add "meals" to the exercise model.
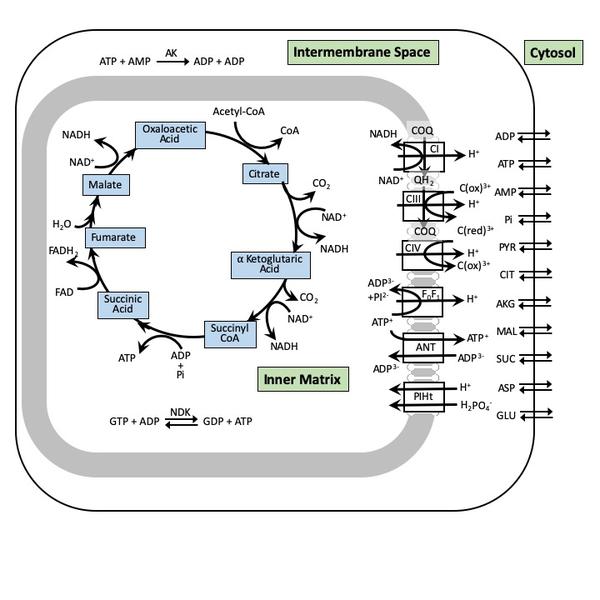 Mitochondrial bioenergeics, metabolism, and aging
Mitochondrial bioenergeics, metabolism, and aging
Bioenergetics is a field in biochemistry and cell biology that concerns energy flow through living systems. How do living organisms acquire and transform energy in order to perform biological work? How do the thousands of different cellular processes, such as cellular respiration and the many other metabolic and enzymatic processes, interact and lead to theproduction and utilization of energy in forms such as adenosine triphosphate (ATP) molecules. The study of metabolic pathways is thus essential to bioenergetics.
Aging, long considered to be solely the result of wear and tear, is in fact regulated by specific genetic and metabolic pathways. We use mathematical modeling to answer important questions: How do simple changes in the environment (e.g. dietary restriction) can drastically extend lifespan? How do several of these signaling pathways control longevity in response to changes in the surroundings?

Circadian rhythms
A circadian rhythm is a roughly 24 hour cycle in the physiological processes of living beings, including plants, animals, fungi and cyanobacteria. There are many examples of circadian rhythms, including the sleep-wake cycle, the body-temperature cycle, and the cycles in which a number of hormones are secreted.
We are particularly interested in the role of the circadian rhythm in diseases and treatment. It has long been recognized that normal glucose‐induced insulin secretion in humans varies across the day–night cycle. How may the disruption of the circadian oscillation of glucose metabolism contribute to the development of type 2 diabetes?
Blood pressure exhibits a circadian rhythm and drops significantly at night time, in what is called a "dipper pattern." Shift workers, however, often show a non-dipper pattern. Does shift work have adverse effects on blood pressure, paritcularly in patients with hypertension? This is an important question, since shift workers make great use of health care services, as they are associated with increased cardiovascular morbidity and mortality.
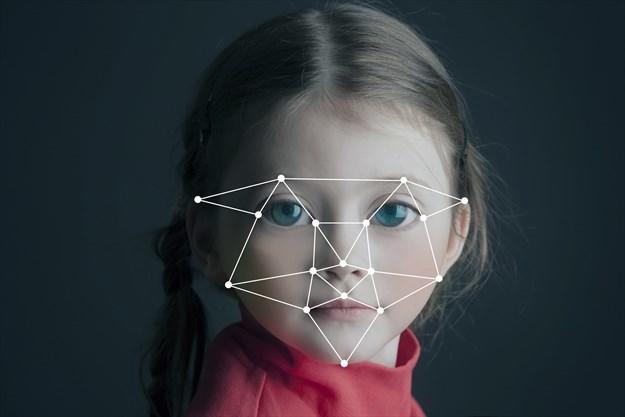 Network analysis of eye gaze patterns
Network analysis of eye gaze patterns
Our group has developed a new autism spectrum disorder (ASD) detection technique that distinguishes different eye-gaze patterns to help doctors more quickly and accurately detect ASD in children. This technique is based on how individuals with ASD visually explore and scan a person’s face differently from neurotypical individuals. An infrared device interprets and identifies the locations on the stimuli at which an individual is looking via emission and reflection of wave from the iris. We then apply network analysis (centrality measures) to evaluate the varying degree of importance the individual placed on different facial features. These measures are significantly different between individuals with ASD and neurotypical individuals.
Eye tracking techniques such as this have many applications, including diagnosing social anxiety, revealing an individual's level of interest (in commercial products, in other people), confidence level, etc.
Data for Good: How to improve water quality rhythms
This is a collaborative project with the Basu lab. Together, we utilize tools from environmental engineering and data science to explore pressing water quality issues. Our research is motivated by these questions: What can we learn from existing datasets? What are the emergent patterns in watershed hydrologic and biogeochemical responses across spatial and temporal scales? Why do these patterns emerge? What are the underlying processes that contribute to these patterns? How do humans interact with the landscapes to modify these patterns in space and time?
The last 100 years have seen a more than threefold increase in global population accompanied by agricultural intensification and dramatic changes in land use. Human activities have greatly accelerated nitrogen (N) and phosphorus (P) cycles, causing excess N and P leaching into surface water that has led to increased algal blooms, eutrophication and drinking water contamination. To mitigate and manage water pollution it is critically important to understand and model the drivers of water quality degradation at regional and global scales. The last decade has seen an exponential increase in the availability of high temporal resolution data from water quality sensors, as well as remote sensing and other spatial datasets, providing exciting opportunities for data scientists and environmental modelers to understand spatially and temporally varying drivers and controls. The objective of the project is to analyze these different datasets, with novel data science and machine learning techniques -- such as Random Forest Models, the Long Short-Term Memory Model (LSTM) that uses a type of Recurrent Neural Network Structure and learns from sequential data -- to undertake detailed multi-scale assessments of drivers of water quality degradation and to confirm generalizable water quality principles at the regional and global scales. Datasets explored will include surface water and groundwater N and P data, remotely sensed chlorophyll-a data in reservoirs and lakes, and soil moisture datasets across the landscape, as well as various land use and land management datasets. Project goal is to integrate these datasets, and develop machine learning models and tools to analyze them.

