The primary focus of the Skeletal Muscle Biology & Cell Death Laboratory is to study cell death processes (i.e., apoptosis and autophagy) in skeletal muscle. In addition, a major theme is the influence of mitochondrial signaling and dynamics on skeletal muscle development, function, regeneration, and health.
The specific areas of focus of the Skeletal Muscle Biology & Cell Death Laboratory currently are:
Autophagic and Mitophagic Signaling in Skeletal Muscle
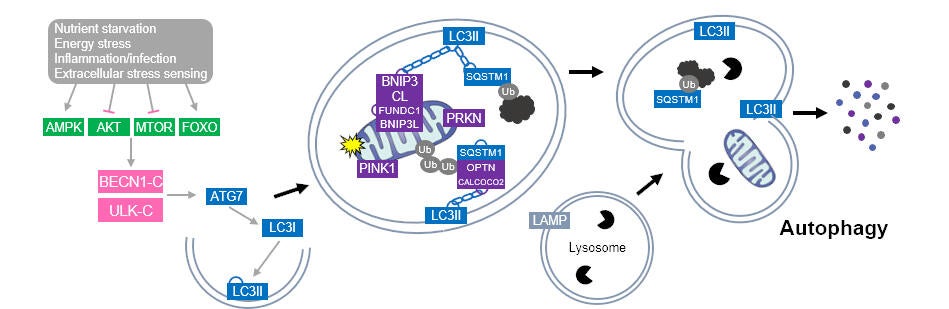
Autophagy is a cellular degradation mechanism that aids in the removal of cytoplasmic contents such as macromolecules and organelles, thereby influencing cell survival (see figure below). It is now well established that dysregulation autophagic signaling play major roles in tissue dysfunction and in the pathogenesis of numerous diseases. The Skeletal Muscle Biology & Cell Death Laboratory is at the forefront of understand the role of autophagic signaling in the formation and maintenance of skeletal muscle. We recently discovered that autophagy is required for skeletal muscle differentiation. Our work has also demonstrated that autophagy and mitophagy (autophagy of mitochondria) are critical in regulating mitochondrial remodeling during muscle formation. Overall, our work has found a critical role for mitophagy and autophagy in mitochondrial quality control and in myogenesis. New work in our lab is examining the influence of autophagy on muscle stem cell function and skeletal muscle regeneration, as well as the role of autophagic signaling on skeletal muscle morphology, adaptation, and function.
Apoptotic Signaling in Skeletal Muscle
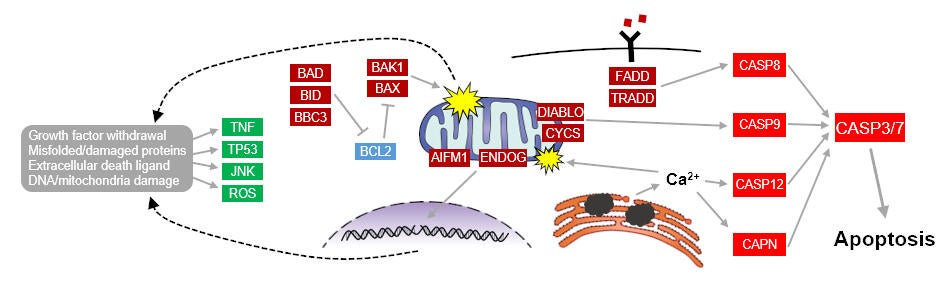
Apoptosis is a highly conserved cell death mechanism that allows multi-cellular organisms to maintain tissue and cellular homeostasis (see figure below). The Skeletal Muscle Biology & Cell Death Laboratory is defining some of the important differences in apoptotic signaling and susceptibility between muscles, fiber types, and mitochondria. We are also investigating the critical role of oxidative stress and mitochondrial content/function on apoptotic susceptibility. Not only are we studying the detrimental effects of apoptotic signaling in skeletal muscle, but we are defining key apoptotic signaling aspects that are essential in skeletal muscle differentiation and formation. For example, classical apoptotic enzymes such as caspases are required for cell differentiation. Recently, our lab discovered that caspase-2 plays an indispensable role in skeletal muscle myogenesis.
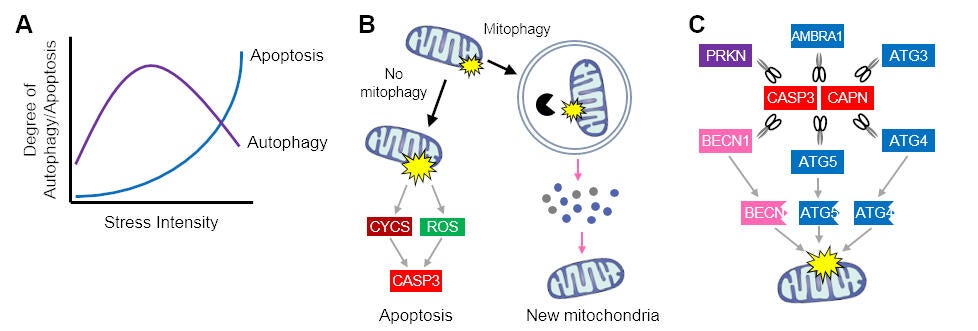
The Skeletal Muscle Biology & Cell Death Laboratory is investigating the interplay between autophagic and apoptotic signaling in skeletal muscle (see figure below). Recently, our lab discovered that autophagy regulates caspase-3 activation during skeletal muscle differentiation, thereby preventing cell death and allowing myogenesis to occur. In addition, our lab has revealed that degradation of damaged and dysfunctional mitochondria through mitophagy protects skeletal muscle cells against cell death during differentiation by preventing mitochondrial-mediated oxidative stress and apoptotic events.
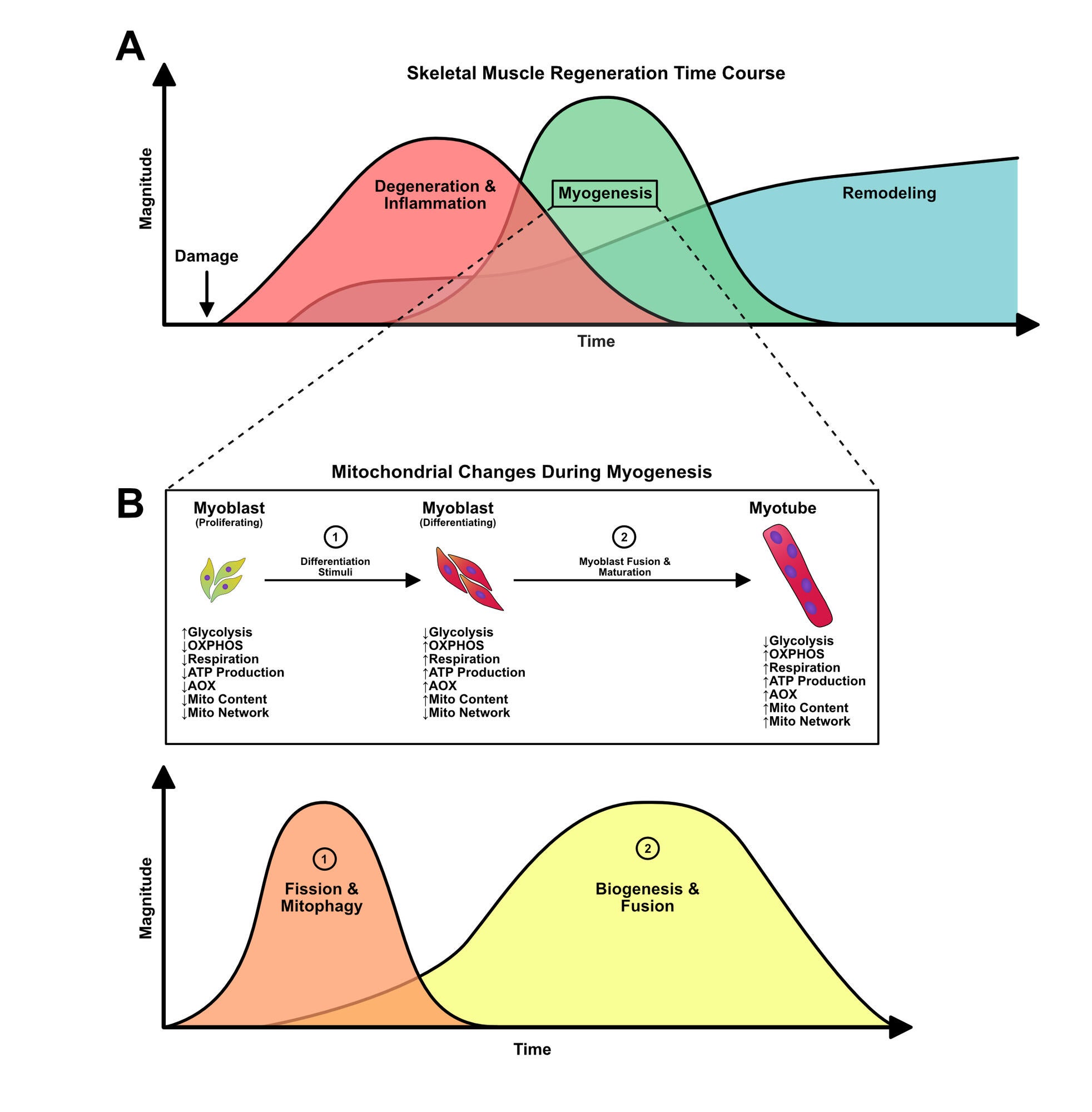
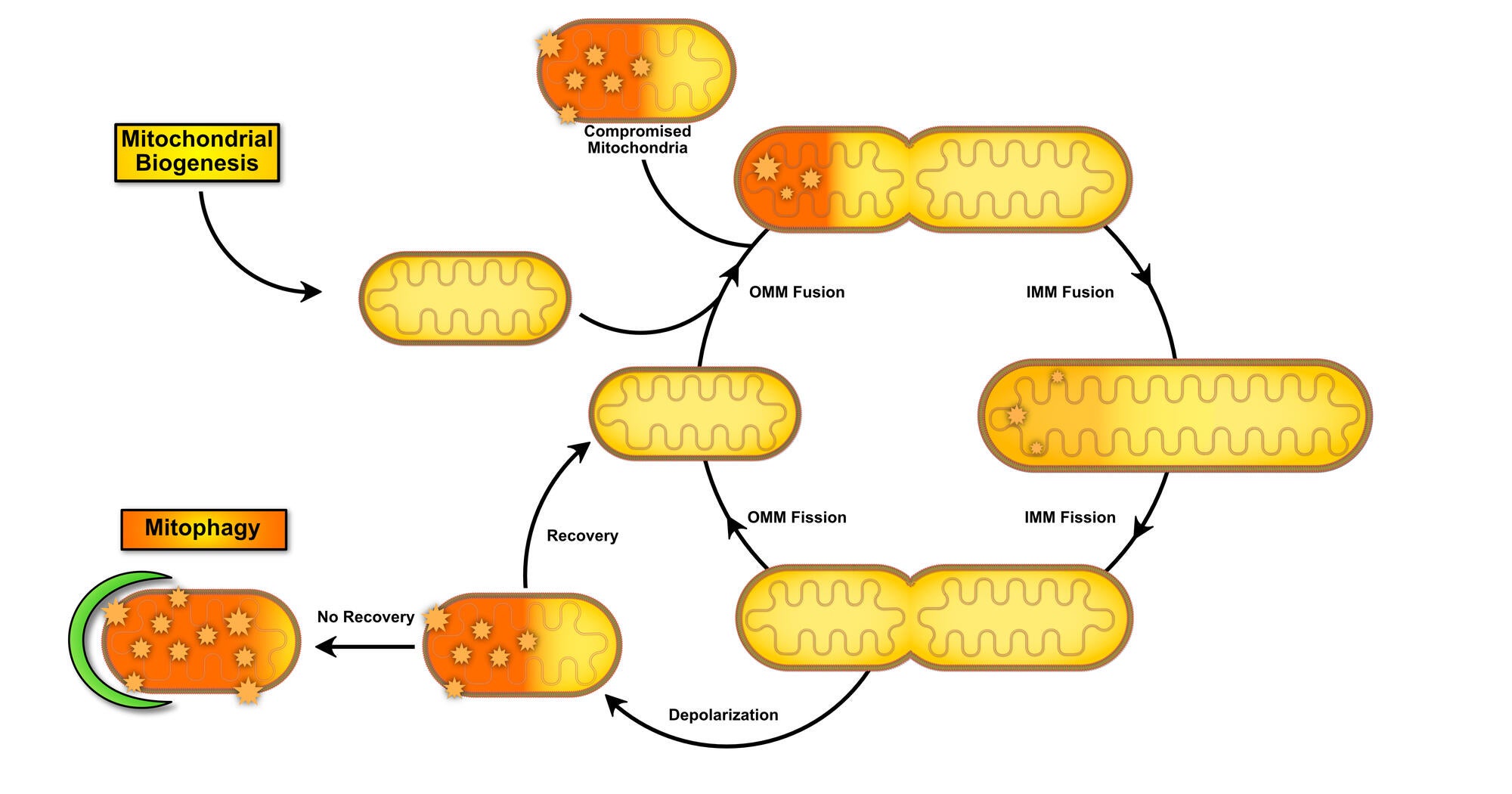
Role of Mitochondrial Dynamics on Skeletal Muscle Regeneration and Myogenesis
Along with mitophagy, mitochondrial biogenesis is important in mitochondrial dynamics by increasing overall mitochondrial content and mass (see figure below). In addition, mitochondrial fission and fusion regulate the specific nature of the mitochondrial network. The Skeletal Muscle Biology & Cell Death Laboratory has found that induction of mitochondrial biogenesis can protect against stress-induced apoptosis, and promote myogenesis in the autophagy-deficient state. In addition, work from our laboratory has found that mitochondrial fission and fusion are required for proper skeletal muscle formation through its influence on apoptotic and autophagic signaling.
 Effect of Physical Activity and Diet on Apoptotic and Autophagic Signaling
Effect of Physical Activity and Diet on Apoptotic and Autophagic Signaling
It is well known that exercise and diet are important in the management and prevention of numerous diseases. Exercise and nutrition influence a number of intracellular signaling pathways. Work from the Skeletal Muscle Biology & Cell Death Laboratory has found that exercise and models that mimic exercise signaling in vitro can alter apoptosis and autophagy in skeletal muscle. New work from our lab is investigating the benefits of acute nutritional interventions on skeletal muscle stem cell autophagic signaling during skeletal muscle damage and regeneration.
