Surface Chemistry of Environmentally-Important Organoarsenic Compounds With Iron (Oxyhydr)oxides Studied by ATR-FTIR, DFT Calculations, and Surface Complexation Modeling
By Dr. Hind Al-Abadleh, Associate Professor, Chemistry, Wilfrid Laurier University
Location: DC1304
Abstract
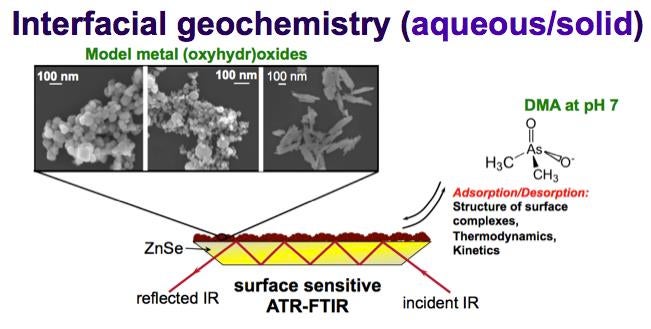
Organoarsenic compounds are found in the environment from the bio-methylation of inorganic arsenic and from anthropogenic sources. They pose health and environmental risks due to their potential cycling to the most toxic forms of arsenic. The environmental fate of arsenic compounds depends on their surface interactions with geosorbents. Molecular-level understanding of the binding mechanism at liquid/solid interfaces demands integrating spectroscopic, computational chemistry and mathematical modeling results. In this talk, I will show ATR-FTIR measurements of the adsorption/desorption behavior of dimethylarsinic acid (DMA) on hematite particles. Experimental results were complemented with DFT calculations of the geometry and IR frequencies of surface clusters, and thermodynamics of ligand exchange reactions. Thermodynamic binding constants were extracted from applying the triple layer surface complexation model to adsorption isotherm and pH-envelope data. The significance of these results will be discussed in relation to improving modeling tools and designing arsenic-removal technologies.
Representative References:
(1) Cowen, S.; Duggal, M.; Hoang, T. N.; Al-Abadleh, H. A., Vibrational Spectroscopic Characterization of Some Environmentally-Important Organoarsenicals: A Guide for Understanding the Nature of Their Surface Complexes. Can. J. Chem. 2008, 86, 942-950.
(2) Tofan-Lazar, J.; Al-Abadleh, H. A., Kinetic ATR-FTIR Studies on Phosphate Adsorption on Iron-(Oxyhydr)Oxides in the Absence and Presence of Surface Arsenic: Molecular-Level Insights into the Ligand Exchange Mechanism. J. Phys. Chem. A 2012, 116, 10143-10149.