Uniform dispersion of inorganic fillers of nano size in organic dielectrics is critical in the development of nanodielectrics and the electrical properties of these materials can be improved if the nanoparticles are well dispersed. One of the problems is that the nanoparticles agglomerate easily because of the high surface energy and conventional mixing techniques do not break apart the nanoparticle agglomerates. Another problem is the incompatibility of a hydrophilic nanoparticles with a hydrophobic polymer such as silicone rubber which results in poor interfacial interactions. Surfactants are being investigated as a way of obtaining a better dispersion of nanoparticles.
Erosion resistance of silicone nanodielectrics
Incline plane tests of silicone rubber composites depict significant resistance to erosion with nano size filler as compared to micron size silica filler with low filler additions (~10% by weight). No improvement in thermal conductivity is evident between nanofilled and unfilled silicone rubber and thermal gravimetric analysis reveals that the bulk properties of both are similar suggesting that the mechanism of protection in nanofilled silicone is different from microfilled silicone rubber.
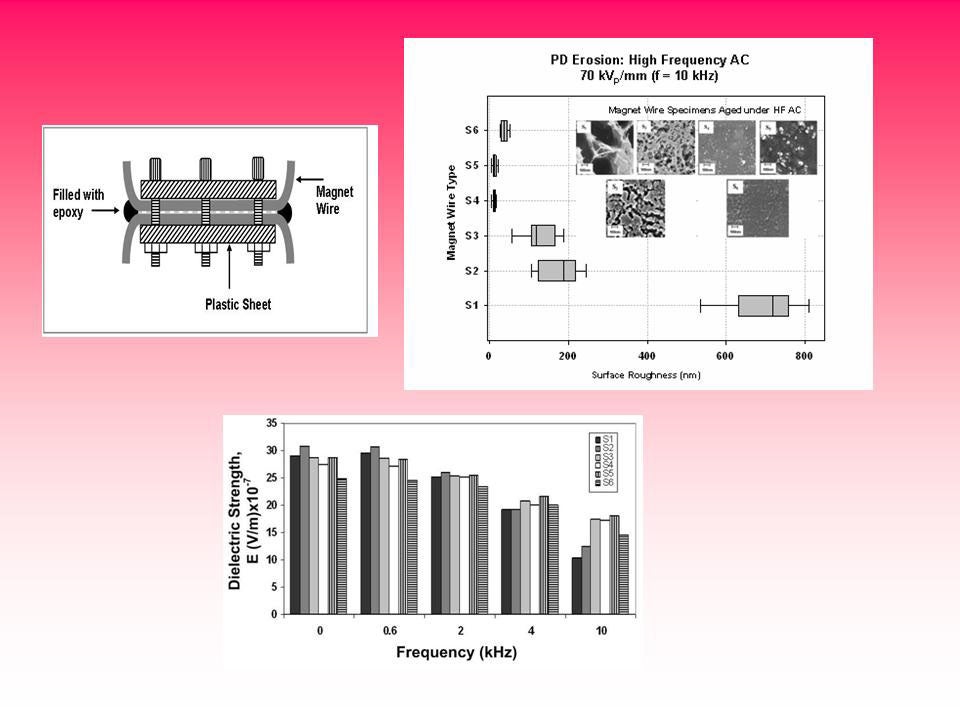
Magnetic wire nanodielectric enamel insulation
Comparative studies on the residual life of magnetic wire enamel insulation under steep-front pulses and high frequency ac waveforms and of newly developed nanofilled insulation containing fumed silica, a nanometer particle, show a significant improvement in the life of ~2.5 times under pulse aging and of ~1.8 times under high frequency waveforms over that of conventional magnet wire insulation. Nanofillers such as TiO2 and Al2O3 show an improved life, however not to the same extent as fumed silica.
Silicone nanodielectrics prepared with surfactant
Surfactants are commonly applied to liquids to aid in particle suspension but their application to disperse nanoparticles in compositions forming nanodielectrics has not yet been reported. Preliminary findings have shown that triton, a common surfactant, significantly aids in the dispersion of nanosilica and nanoalumina, through its hydrophilic character, in silicone rubber, through its hydrophobic component. However, triton cannot interact efficiently with all types of nanofillers and this research is investigating other possible surfactants and methods of dispersing nanoparticles.