Uniform dispersion of inorganic fillers of nano size in organic dielectrics is critical in the development of nanodielectrics and the electrical properties of these materials can be improved if the nanoparticles are well dispersed. One of the problems is that the nanoparticles agglomerate easily because of the high surface energy and conventional mixing techniques do not break apart the nanoparticle agglomerates. Another problem is the incompatibility of a hydrophilic nanoparticles with a hydrophobic polymer such as silicone rubber which results in poor interfacial interactions. Surfactants are being investigated as a way of obtaining a better dispersion of nanoparticles.
Erosion resistance of silicone nanodielectrics
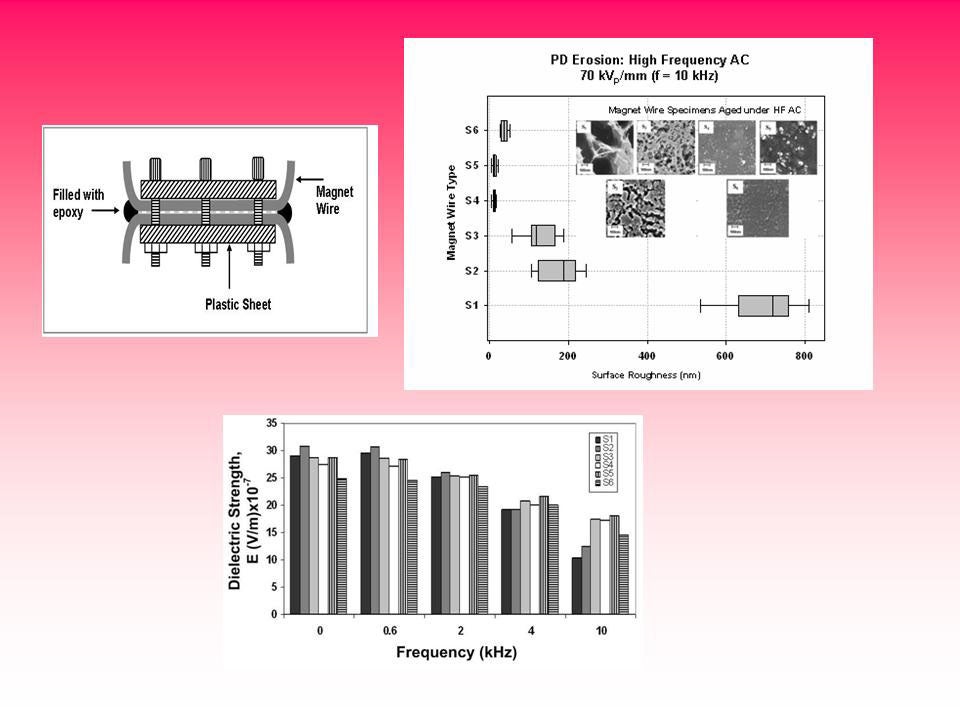
Magnetic wire nanodielectric enamel insulation
Silicone nanodielectrics prepared with surfactant