
Advancing medical imaging
Waterloo spinoff company wins Health Canada licence for innovative X-ray technology

Waterloo spinoff company wins Health Canada licence for innovative X-ray technology
By Brian Caldwell Faculty of EngineeringDigital X-ray technology developed by a University of Waterloo spinoff company has won approval from Health Canada.
A medical device licence issued for Reveal 35C, a dual-energy X-ray detector created by KA Imaging, follows clearance from the Food and Drug Administration in the United States earlier this month.
“Getting this Health Canada licence is a major step forward for KA Imaging, especially coupled with the recent FDA clearance,” Amol Karnick (BASc '95), an engineering alumnus who is president and CEO of the company, said in a media release.
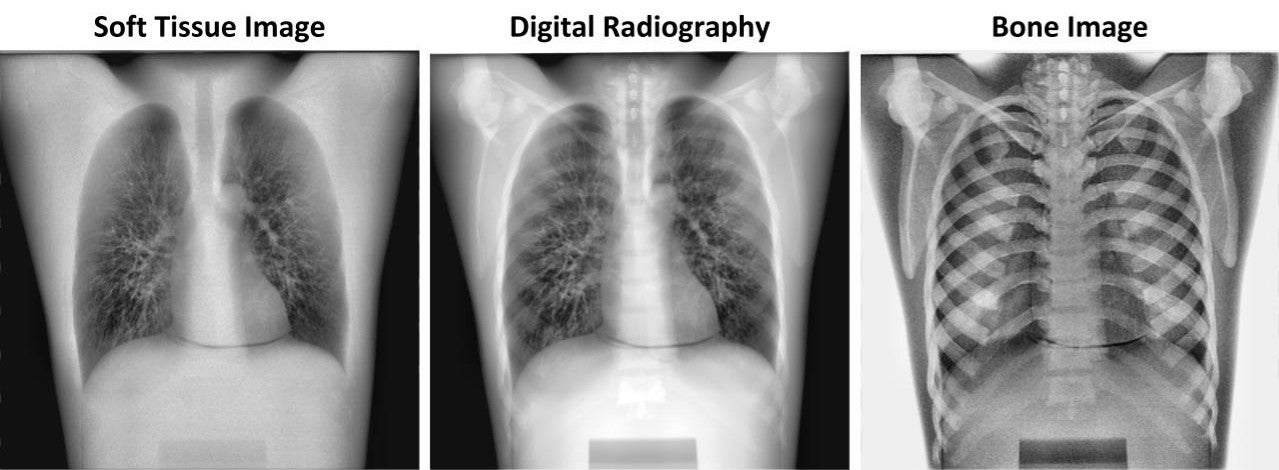
Sample images made with X-ray technology developed by Waterloo startup KA Imaging.
KA Imaging was founded in 2015 by Karnick and fellow alumni Sina Ghanbarzadeh (MASc '14) and Karim S. Karim (BASc '99, PhD '03), a professor of electrical and computer engineering and executive director of the Centre for Bioengineering and Biotechnology at Waterloo.
The company’s flagship product is an inexpensive, portable X-ray detector that can differentiate between bone and soft tissue in a single exposure.
It is now being tested on lung cancer patients at Grand River Hospital in Kitchener and for detection of pneumonia, including cases caused by COVID-19, at a Toronto-based hospital.
Its low cost and portability also make it suitable for use in settings outside hospitals and laboratories.
“At long-term care facilities, which care for vulnerable populations that can suffer from mobility limitations or health concerns, taking the detector to the patient can be very beneficial,” said Karim, chief technology officer of the 25-employee Waterloo company.
In addition to numerous other applications, company officials are hopeful the technology can be used as a triage tool to help prevent the spread of COVID.
Read more
Two University of Waterloo affiliated health-tech companies secure major provincial investment to bring lifesaving innovations to market

Read more
How Doug Kavanagh’s software engineering degree laid the foundation for a thriving career in patient care

Read more
Upside Robotics secures new funding to accelerate the future of sustainable farming
The University of Waterloo acknowledges that much of our work takes place on the traditional territory of the Neutral, Anishinaabeg, and Haudenosaunee peoples. Our main campus is situated on the Haldimand Tract, the land granted to the Six Nations that includes six miles on each side of the Grand River. Our active work toward reconciliation takes place across our campuses through research, learning, teaching, and community building, and is co-ordinated within the Office of Indigenous Relations.