Materials Interface Foundry research profiled by the US Department of Energy
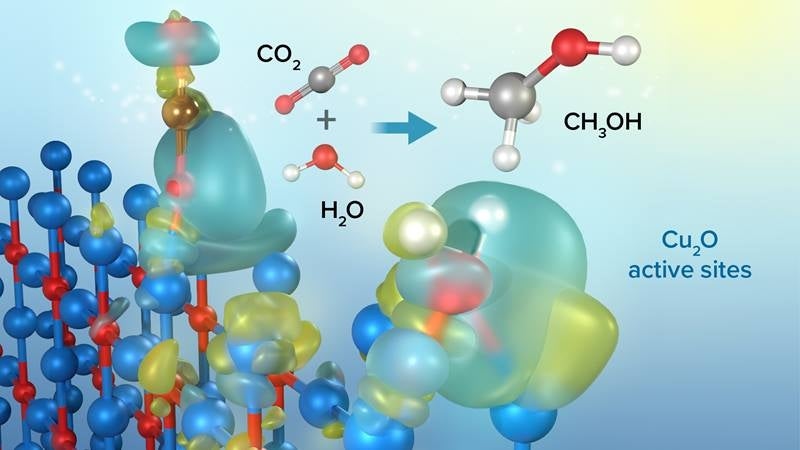
Modelled illustration of copper (I) oxide (Cu2O) photocatalyst particles interacting with carbon dioxide (CO2) and water (H2O) to convert CO2 and water into liquid methanol (CH3OH).
Image courtesy of Argonne National Laboratory
The science
Scientists have revealed the exact structure of a catalyst that transforms carbon dioxide (CO2) and water into liquid fuel in the presence of light. Catalysts are chemicals that accelerate reactions. The researchers used advanced analytical techniques and computer models to study a specific promising catalyst, Copper (I) oxide (Cu2O).
The impact
The efficient conversion of CO2 into liquid fuel could help to reduce our dependence on fossil fuels. Chemical conversion of CO2 using sunlight offers a promising way to store solar energy in the form of liquid chemical fuels. These catalysts that use light are called photocatalysts. Specific catalysts can help facilitate these conversions without unnecessary energy loss. Identifying the active part of catalyst structures, the subject of this study, is an important step in the design of photocatalysts. This ability will help researchers create photocatalysts for converting CO2 into fuels such as methanol and ethylene and for other energy applications.