
New insights into protein structure could change the future of biomedicine
Researchers at the University of Waterloo have discovered a new way to create designer proteins that have the potential to transform biotechnology and personalized medicines

Researchers at the University of Waterloo have discovered a new way to create designer proteins that have the potential to transform biotechnology and personalized medicines
By Media RelationsIn a range of experiments Professor Elizabeth Meiering, in collaboration with colleagues from India and the United States, created a protein that can withstand a range of physiological and environmental conditions - a problem that has challenged chemists looking to create super stable, highly functional proteins.
Their results are published this month in the prestigious, peer-reviewed journal Proceedings of the National Academy of Sciences.
Protein drugs can be engineered to act like antibodies and search out specific cells. Such personalized medicines can attach only where they are needed, greatly reducing side effects in cancer and arthritis treatments. For example, the blockbuster drug Herceptin, currently used to treat several forms of breast cancer, targets HER2 growth receptor sites on the surface of cancer cells and tags the cells so the body’s immune system can destroy them.
Nevertheless, designing a protein that can withstand a range of conditions is challenging and can be risky. Proteins rely on their unique structure to perform their function and one small change to that structure can lead to an allergic reaction, or even a deadly cascade immunological response.
“It’s not enough to design a protein partially right—it needs to be exactly right in order for the protein to be stable and functional and for a drug to work,” said Professor Meiering. “Most natural proteins aren't very stable, and chemists are finding it hard to design stability. Our work represents a real shift in how researchers can engineer and understand proteins.”
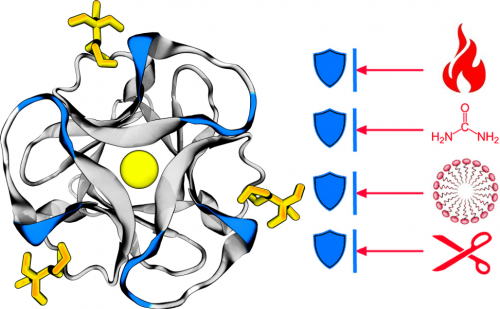
Using extensive experimental and modeling analyses the team show that ThreeFoil, a small glycan binding protein without disulphides (pictured left), exhibits outstanding kinetic stability against chemical denaturation and proteolytic degradation. The "ribbon" (coloured grey and blue) represents the structure of the protein (called "ThreeFoil"). In yellow are "ligands" to which ThreeFoil specifically binds functionally. In red are stresses that ThreeFoil is resistant against: (from top to bottom) heat or high temperature, chemical denaturants, detergents, and proteases.
Traditionally protein designers focus on either structure or function because working with each property is challenging in its own right.
Professor Meiering and her colleagues were able to incorporate both structure and function into the design process by using bioinformatics to leverage information from nature. They then analyzed what they made and measured how long it took for the folded, functional protein to unfold and breakdown.
“A protein can be energetically unstable, yet still unfold extremely slowly—meaning it remains folded and functional for a long time,” said Professor Meiering, a member of the Centre for Bioengineering and Biotechnology.
Using a combination of biophysical and computational analyses, the team discovered this kinetic stability can be successfully modeled based on the extent to which the protein chain loops back on itself in the folded structure. Because their approach to stability is also quantitative, the protein’s stability can be adjusted to naturally break down when it is no longer needed.
This different way of thinking will allow researchers to move forward with designing proteins with precisely controlled stability for challenging applications such as biosensors and personalized therapeutics.
University of Waterloo co-authors include PhD student Aron Broom, former MSc student Martha Ma and PhD student Kyle Trainor. International collaborators include members from the Gosavi group at the Tata Institute of Fundamental Research in Bangalore, India and the Colón group at Rensselaer Polytechnic Institute in New York.

Read more
The Government of Canada announces funding to support research in food policies and medical devices

Read more
Waterloo prof leads a team of researchers to improve water quality through a community-focused approach underpinned by technical excellence

New research examines the carbon-removal potential of strategic planting (Getty Images/Zhao Qin).
Read more
AI-powered modelling shows planting in northern forests could help Canada become carbon neutral by mid-century
The University of Waterloo acknowledges that much of our work takes place on the traditional territory of the Neutral, Anishinaabeg, and Haudenosaunee peoples. Our main campus is situated on the Haldimand Tract, the land granted to the Six Nations that includes six miles on each side of the Grand River. Our active work toward reconciliation takes place across our campuses through research, learning, teaching, and community building, and is co-ordinated within the Office of Indigenous Relations.