Background
One of the fastest growing ophthalmic pharmaceutical market segments, the anterior dry eye disease therapeutic market is predicted to grow at an annual rate of 10% from 2010 to 2014. By 2015, this market is expected to exceed $1 billion USD. Common topical formulations, such as eye drops or ointments, suffer from low ocular bioavailability due to rapid drainage through the nasolacrimal duct, nearly constant dilution by tear turnover, and low drug permeability across the corneal epithelium. As a result, topical formulations are normally administered multiple times daily in order to achieve therapeutic efficacy, resulting in a higher potential for side effects and lower patient compliance. Eye drop solutions undergo rapid clearance from the eye surface due mainly to tear turnover, causing rapid clearance of 95% of administered drugs within 10 minutes upon delivery. Drugs must therefore be administered frequently which in turn lowers patient compliance and increases undesirable side effects.
Description of the invention
This technology is designed to overcome the rapid clearance of current eye drop solutions thereby improving drug retention on the corneal surface. The technology uses nano-scaled drug carriers, their biocompatibility profile has been well accepted in clinics, and their degradability profile can be controlled between several days to 6 weeks in human tissue. The technology does not significantly affect the transparency or the solution viscosity, which improves patient compliance. The surfaces of the nanoparticles have a defined number of ligands capable of targeting the corneal surface, allowing drugs encapsulated in the particles to effectively circumvent the tear turnover mechanism, and significantly improving their corneal retention.
Advantages
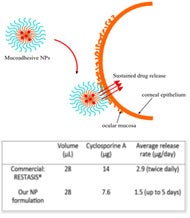
To demonstrate proof-of-concept, we showed the eye drop formulation can deliver a therapeutically relevant dosage of Cyclosporine A, a drug commonly administered in the treatment of dry eye syndrome. Release of the particles was sustained for up to 5 days, which suggests that the current formulation may significantly reduce administration frequency without compromising the therapeutic efficacy of the drug. We also showed that the nanoparticle can selectively and efficiently bind to the outer structural layer of the corneal surface during the duration of release.
Potential applications
This technology may deliver a wide range of bioactive agents including both hydrophilic and hydrophobic compounds. The technology may serve as a platform technology to provide long lasting delivery of therapeutic agents to treat anterior eye diseases.
For further details and impact, see Waterloo news.
Long-lasting eye drop delivery platform for targeted ocular delivery applications
Reference
7341
Patent status
Patent issued
Stage of development
Prototype and validation
Contact
Scott Inwood
Director of Commercialization
Waterloo Commercialization Office
sinwood@uwaterloo.ca
uwaterloo.ca/research