UNIVERSITY OF WATERLOO
DEPARTMENT OF CHEMISTRY
15 MAY 2008
TIME: 75 MINUTES
This exam is being written by several thousand students. Please be sure that you follow the instructions below.
We'll send your teacher a report on your performance. Top performers are eligible for a prize. The names of the top 200 students will be published in the September issue of Chem 13 News.
- Print your name here: _________________________
- Print your school name and city on your STUDENT RESPONSE sheet.
- Select, and enter on the STUDENT RESPONSE sheet, one of the following CODE numbers:
- Code 1 Ontario, now studying Grade 12 Chemistry in a nonsemestered school
- Code 2 Ontario, now studying Grade 12 Chemistry in a semestered school
- Code 3 Ontario, Grade 12 Chemistry already completed
- Code 4 Any other Ontario student
- Code 5 Manitoba or Saskatchewan high school student
- Code 6 Québec high school student
- Code 7 Québec CEGEP student
- Code 8 Alberta or British Columbia high school student
- Code 9 New Brunswick, Newfoundland, Nova Scotia, or Prince Edward Island high school student
- Code 10 Northwest Territories, Nunavut, or Yukon high school student
- Code 11 High school student outside Canada
- Code 12 Teacher
- Print your name (last name, first name and optional middle initial) on the STUDENT RESPONSE sheet. Also fill in the corresponding circles below your printed name.
- Carefully detach the last page. It is the datasheet.
- Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number.
- Mark only one answer for each question.
- Questions are all of the same value.
- There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer.
- Take care that you make firm, black pencil marks, just filling the oval.
Be careful that any erasures are complete—make the sheet white again.
Carefully detach the last page. It is the Data Sheet.
1. Which of the following elements is not a metal?
- Se
- Sn
- Sr
- Sc
- Cs
2. A colourless, odourless gas is thought to be oxygen.
Which of the following experimental results would support this conclusion?
- Burning the gas in air produces only water.
- The gas extinguishes a flame.
- The gas turns a Ca(OH)2 solution milky.
- A glowing piece of wood bursts into flames in the gas.
- The gas tarnishes silver.
3. Which of the following particles is the most massive?
- α-particle
- β-particle
- electron
- proton
- neutron
4. What volume of 5.0 mol L⁻¹ H₂SO₄(aq) must be diluted with water to make 1.00 L of 0.45 mol L⁻¹ H₂SO₄(aq)?
- 0.090 L
- 0.44 L
- 0.090 mL
- 0.045 L
- 2.22 mL
5. How many neutrons are there in the nucleus of ¹³¹I?
- 44
- 53
- 78
- 131
- 184
6. Which group of elements contains no metals or metalloids?
- group 13
- group 14
- group 15
- group 16
- group 17
7. Which of these chloride salts is least likely to exist?
- NaCl
- CuCl
- CaCl₂
- FeCl₃
- MgCl
8. When a sample of atomic hydrogen gas is heated, it emits violet, blue, green and red light.
Which of the following statements best explains this observation?
- The energy of the electron in a hydrogen atom is restricted to certain values.
- The energy of the electron in a hydrogen atom is not restricted in any way.
- The electron in a hydrogen atom is restricted to one of only four possible circular orbits.
- The distance between the electron and the nucleus in a hydrogen atom is restricted to certain values.
- none of the above
9. Which of the following is not a mixture?
- seawater
- table sugar
- brass
- cement
- smoke
10. Radioactive 131I is used to treat thyroid cancer.
An incomplete chemical equation for the radioactive decay of 131I is given below.
131I → ? + 0-1e
What is the missing product in the equation above?
- 130I
- 129I
- 131Xe
- 131Te
- 131I+
11. Which of the following has the highest concentration in air at STP?
- He
- H2O
- CO2
- N2
- O2
12. The average mass of a solid copper penny is 2.63 g.
What is the mass of one mole of pennies?
- 1.58×1024 g
- 6.02×1023 g
- 6.36×1023 g
- 63.6 g
- 1.58×1023 g
13. What is the sum of the coefficients when the following equation is balanced using the smallest whole number coefficients?
P + Cl2 → PCl3
- 12
- 11
- 6
- 5
- 3
14. How many litres of gaseous methane (CH4) must be burned in oxygen to produce enough H2O and CO2 to fill a 3.0-L balloon?
Assume that H2O and CO2 are the only combustion products and that the temperature and pressure remain constant.
- 1.0 L
- 1.5 L
- 2.0 L
- 2.5 L
- 3.0 L
15. A compound that contains only Fe and O is 69.9% Fe by mass.
What is the empirical formula of this compound?
- FeO
- FeO2
- Fe2O3
- Fe2O
- Fe3O4
16. If 17.0 grams of sodium chloride are dissolved in water to make 0.5 L of solution, then what is the final concentration of the solution?
Give your answer with the correct number of significant figures.
- 0.6 mol L-1
- 0.58 mol L-1
- 0.581 mol L-1
- 0.3 mol L-1
- 0.291 mol L-1
17. What is the effect of adding a catalyst to a reaction mixture?
- It increases the equilibrium concentrations of the products.
- It decreases the enthalpy change of the reaction.
- It reduces the activation energy of the reaction.
- It increases the value of the equilibrium constant for the reaction.
- It increases the time it takes for the reaction to reach equilibrium.
18. How many valence electrons are there in one Al3+ ion?
- 2
- 4
- 6
- 8
- 10
19. What volume of He(g) contains the same number of moles of gas as 1.00 L of N2(g) at the same temperature and pressure?
- 7.00 L
- 1.00 L
- 0.143 L
- 35.7 mL
- 4.00 L
20. What is the HNH bond angle in an ammonia (NH3) molecule?
Choose the closest value.
- 90°
- 45°
- 120°
- 109°
- 180°
21. Which of the following types of radiation has the lowest energy per photon?
- radio waves
- ultraviolet radiation
- infrared radiation
- x-rays
- purple laser light
22. An incomplete Lewis structure (i.e. electron dot structure) for the O3 molecule is given below.
O—O—O
How many lone pairs of electrons are there in the completed structure?
- two
- four
- five
- six
- eight
23. Which of the following is not a common oxide of nitrogen?
- NO
- NO2
- N2O4
- N2O
- NO3
24. In an experiment, 0.12 L of 0.10 mol L-1 H2SO4(aq) and 0.20 L of 0.10 mol L-1 NaOH(aq) are combined.
Which of the following statements is true?
- The pH of the resulting solution is less than 7.
- The pH of the resulting solution is greater than 7.
- The pH of the resulting solution is close to 7.
- The pH of the resulting solution is exactly 7.
- None of the statements above are true.
25. Solid aluminum dissolves in hydrochloric acid solution according to the following chemical equation.
2 Al(s) + 6 HCl(aq) → 2 AlCl3(aq) + 3 H2(g)
How many moles of H2 are produced if 17.5 moles of Al are added to a solution containing 24.8 moles of HCl?
- 26.3 mol
- 12.4 mol
- 7.30 mol
- 17.5 mol
- 24.8 mol
26. Which of the following choices does not involve a chemical change?
- evaporation and neutralization
- neutralization and sublimation
- oxidation and sublimation
- evaporation and sublimation
- neutralization and oxidation
27. Which of the following atoms or ions has the electron configuration 1s22s22p63s1 in its ground electronic state?
- Na
- Mg+
- K
- Ca+
- Al3+
28. Which of the following is a brittle solid and an electrical insulator at room temperature, but an excellent electrical conductor in its liquid form?
- sulphur
- sodium chloride
- aluminum
- mercury
- carbon
29. Which of the following salts produces a basic solution when it is dissolved in water?
- KCl
- NH4Cl
- K2CO3
- NaNO3
- CuBr2
30. Which of the following describes the process that produces Fe(s) from Fe2O3(s)?
- combustion
- precipitation
- hydrolysis
- reduction
- oxidation
31. Which one of the following solutions will be the worst electrical conductor at 25°C?
- 0.10 mol L-1 Na2SO4(aq)
- 0.10 mol L-1 NaCl(aq)
- 0.10 mol L-1 CaSO4(aq)
- 0.10 mol L-1 CH3OH(aq)
- 0.10 mol L-1 CsCl(aq)
32. Which of the following atoms is not present in large numbers in biological molecules?
- C
- F
- O
- N
- H
33. In which of these compounds is the oxidation state of Cl the highest?
- HClO2
- ClO2
- Cl2O5
- Cl2O
- HClO4
34. Which of the gases most closely resembles an ideal gas at standard temperature and pressure?
- CO2
- NH3
- HI
- H2
- H2O
35. Which of the following have ground state electron configurations of the type ns2np2?
- group 2 atoms
- group 4 atoms
- group 6 atoms
- group 14 atoms
- group 16 atoms
36. Which of the species in the reaction below are Brønsted-Lowry acids?
HSO4- + HCO3- ⇌ SO42- + H2CO3
- HSO4- and HCO3-
- HSO4- and H2CO3
- HCO3- and SO42-
- SO42- and H2CO3
- HSO4- and SO42-
37. Which of the following is not an alkane?
- C2H4
- C3H8
- C4H10
- C5H12
- C6H14
38. What happens when a solution of lithium chloride (LiCl) and a solution of ammonium nitrate (NH₄NO₃) are mixed?
- A precipitate forms.
- A new salt is formed.
- A gas is evolved.
- A metal is formed.
- No reaction occurs.
39. An average person expends approximately 100 kJ to walk 1 km.
How far will the average car travel by the time it expends the same amount of energy (i.e. 100 kJ) as a person who walked 1 km?
Use the data given below to determine the answer.
Choose the closest answer.
-
Fuel consumption of an average car, 8 km L⁻¹
-
Heat of combustion of gasoline, 50 kJ g⁻¹
-
Density of gasoline, 0.7 g mL⁻¹
- 2 km
- 0.2 km
- 0.02 km
- 20 km
- 200 km
40. How many structural isomers are there for C6H12?
- less than three
- three
- four
- five
- more than five
AVOGADRO EXAM 2009 DATA SHEET
DETACH CAREFULLY
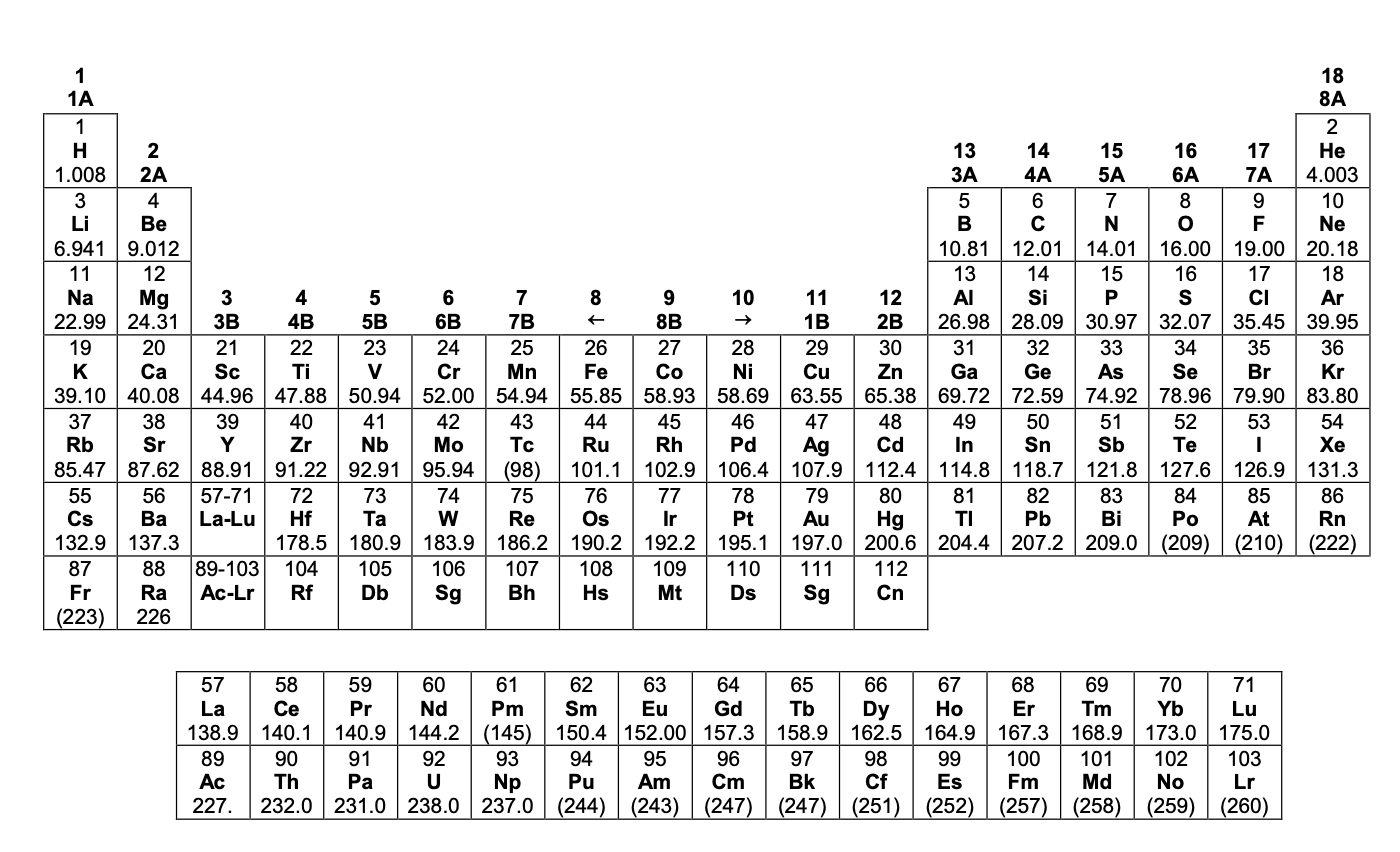
Additional interactive periodic tables
Constants
NA = 6.022 x 1023 mol-1
R = 0.08206 atm L K-1 mol-1 = 8.3145 kPa L K-1 mol-1 = 8.3145 J K-1 mol-1
Kw = 1.0 \times 10^{-14} (at 298 K)
F = 96 485 C mol-1
Conversion factors
1 atm = 101.325 kPa = 760 torr = 760 mm Hg
0oC = 273.15 K
Equations
PV = nRT
k t1/2 = 0.693
pH = pKa + log ([base]/[acid])
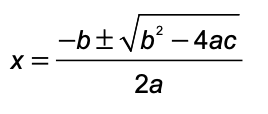
CHEM 13 NEWS EXAM © 2011 UNIVERSITY OF WATERLOO