UNIVERSITY OF WATERLOO
DEPARTMENT OF CHEMISTRY
20 MAY 2010
TIME: 75 MINUTES
This exam is being written by several thousand students. Please be sure that you follow the instructions below.
We'll send your teacher a report on your performance. Top performers are eligible for a prize. The names of the top 200 students will be published in the September issue of Chem 13 News.
- Print your name here: _________________________
- Print your school name and city on your STUDENT RESPONSE sheet.
- Select, and enter on the STUDENT RESPONSE sheet, one of the following CODE numbers:
- Code 1 Ontario, now studying Grade 12 Chemistry in a nonsemestered school
- Code 2 Ontario, now studying Grade 12 Chemistry in a semestered school
- Code 3 Ontario, Grade 12 Chemistry already completed
- Code 4 Any other Ontario student
- Code 5 Manitoba or Saskatchewan high school student
- Code 6 Québec high school student
- Code 7 Québec CEGEP student
- Code 8 Alberta or British Columbia high school student
- Code 9 New Brunswick, Newfoundland, Nova Scotia, or Prince Edward Island high school student
- Code 10 Northwest Territories, Nunavut, or Yukon high school student
- Code 11 High school student outside Canada
- Code 12 Teacher
- Print your name (last name, first name and optional middle initial) on the STUDENT RESPONSE sheet. Also fill in the corresponding circles below your printed name.
- Carefully detach the last page. It is the datasheet.
- Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number.
- Mark only one answer for each question.
- Questions are all of the same value.
- There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer.
- Take care that you make firm, black pencil marks, just filling the oval.
Be careful that any erasures are complete—make the sheet white again.
Carefully detach the last page. It is the Data Sheet.
1. Of the first 18 elements, how many are gases at 25°C and 100 kPa?
- less than seven
- seven
- eight
- nine
- more than nine
2. Which of the following substances has the highest vapour pressure at 25°C?
- CH3OH
- CH3CH2CH2OH
- LiF
- H2CO
- Li
3. Which of the following compounds has the highest melting point?
- LiF
- ZnO
- LiCl
- NaF
- NaCl
| Li+ | 68 | F- | 136 |
|---|---|---|---|
| Zn2+ | 74 | O2- | 140 |
| Na+ | 97 | Cl- | 181 |
4. For a given substance, which of the following phase transitions is the most exothermic?
- solid → liquid
- gas → liquid
- liquid → gas
- solid → gas
- gas → solid
5. What is the ground state electron configuration of selenium, Se?
- 1s2 2s2 2p6 3s2 3p6 4s2 4p4
- 1s2 2s2 2p6 3s2 3p6 3d10 4p6
- 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p4
- 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6
- 1s2 2s2 2p6 3s2 3p6 4s2 4p4 4d10
6. The reaction below reaches equilibrium in a closed reaction vessel.
C6H12O6(s) ⇌ 2 C2H5OH(l) + 2 CO2(g), ΔH° = -72 kJ
Which of the following actions causes an increase in the value of Kc?
-
adding some CO2(g)
-
transferring the reaction mixture to a vessel of larger volume
-
increasing the temperature
- (i) only
- (ii) only
- (iii) only
- (i) and (ii)
- none of the above
7. Given that
2 Hg2+(aq) + 2 e- ⇌ Hg22+(aq) E° = 0.920 V
Ag+(aq) + e- ⇌ Ag(s) E° = 0.799 V
what is E° for the reaction below?
2 Ag+(aq) + Hg22+(aq) → 2 Ag(s) + 2 Hg2+(aq)
- 0.121 V
- -0.121 V
- 0.678 V
- -0.678 V
- 0.339 V
8. Given that
Fe2+(aq) + 2 e- ⇌ Fe(s) E° = -0.40 V
2 H+(aq) + 2 e- ⇌ H2(g) E° = 0.00 V
Br2(l) + 2 e- ⇌ 2 Br-(aq) E° = +1.09 V
which of the following is the strongest reducing agent under standard conditions?
- Fe2+(aq)
- H+(aq)
- Br2(l)
- Br-(aq)
- H2(g)
9. What is the coefficient of O2 when the following equation is balanced?
1 C10H6(s) + x O2(g) → y CO2(g) + z H2O(l)
10. Which of the following will react appreciably with water at room temperature and pressure to produce hydrogen?
11. Cesium forms a number of compounds with oxygen. A particular compound is found to be 26.5% oxygen by mass. What is the formula of this compound?
Molar masses (in g/mol)
O, 16.00
Cs, 132.9
12. Which of the following is the strongest acid in water?
13. Let the energy of the 2s level in a hydrogen atom be −E.
What is the energy of the 3s level?
14. Natural oils, such as vegetable oil, are converted into solid, edible fats by a process called
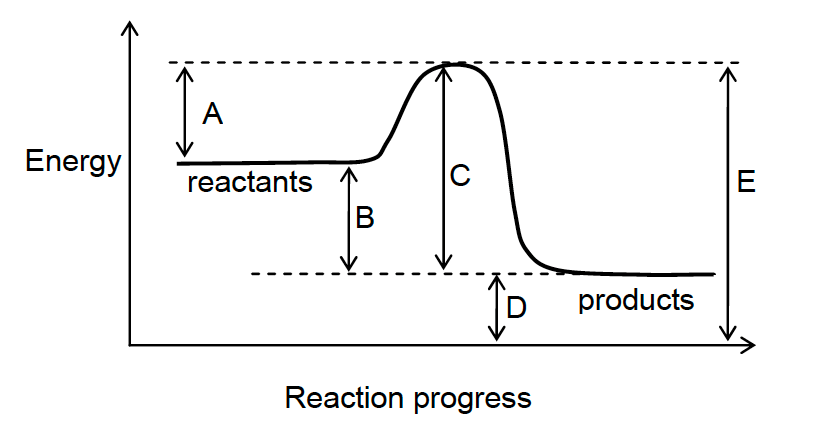
15. The value for the activation energy of the forward reaction is represented by which letter in the diagram below?
16. The heat of combustion of C(s) is -394 kJ/mol and that of CO(g) is -111 kJ/mol.
What is the enthalpy change reaction below?
CO(g) → C(s) + ½O2(g)
- 505 kJ
- 283 kJ
- 111 kJ
- -283 kJ
- -505 kJ
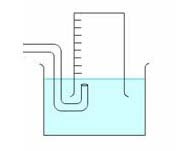
17. Exactly 850 mL of O2 gas is collected over water at 30.0°C using the setup below.
Given that the barometric pressure was 98.5 kPa and the vapour pressure of water is 4.24 kPa at 30°C,
what volume would the pure O2 gas occupy at 98.5 kPa and 30°C?
- 813 mL
- 818 mL
- 850 mL
- 882 mL
- 888 mL
18. How are the boiling and freezing points of water affected by the addition of a soluble salt?
- The freezing and boiling points are both lowered.
- The freezing and boiling points are both raised.
- The freezing is lowered and the boiling point is raised.
- The freezing is raised and the boiling point is lowered.
- The boiling and freezing points are not affected.
19. The reaction below comes to equilibrium in a closed reaction vessel of volume 2.50 L.
2 NO2(g) ⇌ 2 NO(g) + O2(g)
At equilibrium, there are 3.0 mol NO, 4.00 mol O2 and 22.0 mol NO2.
What is the value of Kc for the reaction above?
- 0.0298
- 33.6
- 1.83
- 13.4
- 0.218
20. Which of the following occurs if a 0.10 mol/L solution of a weak acid is diluted to 0.010 mol/L at constant temperature?
- The hydrogen ion concentration decreases to 0.010 mol/L.
- The pH decreases.
- The ionization constant, Ka, decreases.
- The percentage ionization increases.
- all of the above
21. What is the equilibrium concentration of Ag+ in solution when 0.50 L of 0.10 mol/L AgNO3(aq) and 0.50 L of 0.20 mol/L NaCl(aq) are mixed?
Assume the temperature is 25°C; For AgCl, Ksp = 1.8×10-10 at 25°C.
- 0 mol/L
- 3.6×10-9 mol/L
- 9.0×10-10 mol/L
- 1.3×10-5 mol/L
- 0.05 mol/L
22. In which ionic compound does the cation have the same number of electrons as the anion?
- LiF
- NaCl
- CaO
- MgF2
- KI
23. How many moles of NaOH or HCl should be added to 1.0 L of 0.010 mol L-1 formic acid (HCOOH) solution to obtain a solution with pH = 3.50?
Assume no change in volume. (Choose the closest value.)
Ka = 1.8×10-4 for HCOOH
- 3.6×10-3 mol NaOH
- 3.6×10-3 mol HCl
- 5.8×10-3 mol NaOH
- 5.8×10-3 mol HCl
- 3.2×10-4 mol HCl
24. For the reaction below,
Kc = 6.3×104 at 25°C. 2 NO(g) + Cl2(g) ⇌ 2 NOCl(g)
In an experiment, carried out at 25°C:
1.0 mol NO and 1.0 mol Cl2 are added to an evacuated reaction vessel of volume 1.0 L and then the vessel is quickly sealed.
What is the equilibrium concentration of NO?
- 0.50 mol/L
- 5.6×10-3 mol/L
- 2.8×10-3 mol/L
- 1.6×10-9 mol/L
- 7.9×10-6 mol/L
25. What is the molecular geometry of the BrF3 molecule?
The Br atom is the central atom and all the F atoms are bonded directly to Br.
- trigonal planar
- trigonal bipyramidal
- T-shaped
- square planar
- trigonal pyramidal
26. When 0.012 moles of a monoprotic acid is dissolved in water to give 1.0 L of solution at 25°C, the final pH is 1.95.
What is Ka for this acid?
- 2.9×10-1
- 1.1×10-2
- 1.6×10-3
- 1.3×10-4
- 1.5×10-6
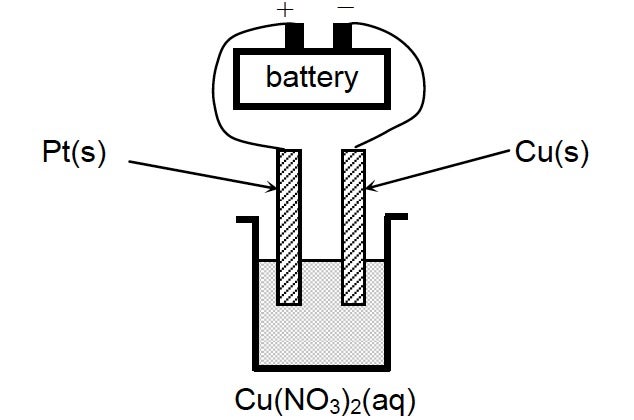
27. A 1.00 mol/L solution of Cu(NO3)2(aq) is electrolyzed using the setup illustrated.
What is the reaction occurring at the anode?
- Cu2+(aq) + 2e- → Cu(s)
- Cu(s) → Cu2+(aq) + 2 e-
- 2 H2O(l) + 2 e- → H2(g) + 2 OH-(aq)
- 2 H2O(l) → O2(g) + 4 H+(aq) + 4 e-
- Pt(s) → Pt4+(aq) + 4 e-
28. Which of the following forms of radiation has the longest wavelength?
- infrared
- x-ray
- microwave
- ultraviolet
- visible
29. In the unbalanced chemical equation below, x, y and z are coefficients to be determined.
1 Fe2+ + x Br2 → y Fe3+ + z Br-
When the equation is properly balanced, what is the value of z ?
- 1
- 2
- ½
- 4
- ¼
30. If the pH of a solution changed from 4.0 to 8.0, what happened to the hydrogen ion concentration?
- It increased by a factor of two.
- It decreased by a factor of two.
- It increased by a factor of 104.
- It decreased by a factor of 104.
- It decreased by a factor of 102.
31. Which of the following compounds displays only covalent bonding?
- NH4OH
- Li2O
- HOCN
- NaNO3
- KH
32. How many sigma (σ) and pi (π) bonds are there in the allene molecule, H2CCCH2?
- six σ bonds and two π bonds
- two σ bonds and six π bonds
- four σ bonds and four π bonds
- eight σ bonds and no π bonds
- two σ bonds and six π bonds
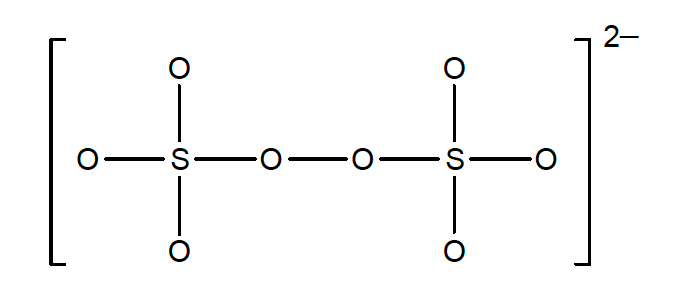
33. What is the oxidation state of each sulfur atom in the peroxydisulfate ion, S2O82-? In the structure below, lone pairs are not shown.
- -2
- 0
- +4
- +6
- +7
34. A Lewis structure for POCl3 is shown below. Which of the following statements is correct?
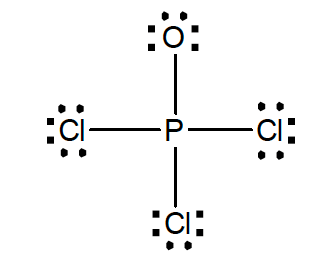
- This is the most important Lewis structure for the POCl3 molecule.
- The phosphorus atom is sp2 hybridized.
- The Cl-P-Cl angles are 90°
- The oxidation state of phosphorus is +4.
- None of the statements above are true.
35. What is the maximum number of electrons that can have a principal quantum number of 4 within one atom?
- two
- four
- eight
- sixteen
- thirty-two.
36. How many unpaired electrons are there in a Mn2+ ion in its ground electronic state?
The atomic number of manganese is Z = 25.
- 0
- 2
- 3
- 5
- 6
37. The skeletal structure below for the CH2CHOCN molecule is incomplete; additional bonding pairs or lone pairs must be added.
When the structure is properly completed, how many lone pairs are there in this molecule?
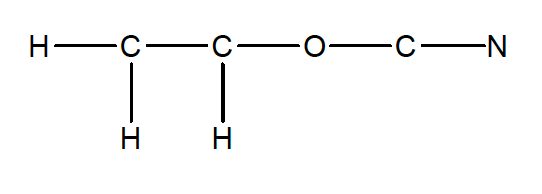
- none
- one
- two
- three
- four
38. When temperature is increased, the rate of a reaction also increases.
This observation is best explained by
- an increase in the frequency of molecular collisions
- a decrease in the activation energy, Ea, for the reaction
- an increase in the activation energy, Ea, for the reaction
- a decrease in the enthalpy change, ΔH, for the reaction
- an increase in the fraction of molecules that have enough energy to react
39. Which of the following would need the smallest quantity of heat to change the temperature of 5 g by 10°C?
| Specific Heat (in J g-1 °C-1) | |
| I2(s) | 0.143 |
| H2O(l) | 4.18 |
| Au(s) | 0.129 |
| He(g) | 5.19 |
| Cu(s) | 0.385 |
- I2(s)
- H2O(l)
- Au(s)
- He(g)
- Cu(s)
40. Let HA represent a weak monoprotic acid with Ka = 1.0×10-5.
What is the pH at the equivalence point in the titration of 50.0 mL of 0.20 mol/L HA(aq) with 0.20 mol/L NaOH(aq)?
- 5.00
- 9.00
- 7.00
- 3.00
- 11.00
CHEM 13 NEWS EXAM 2010 DATA SHEET
DETACH CAREFULLY
Additional interactive periodic tables
Constants
NA = 6.022 x 1023 mol-1
R = 0.08206 atm L K-1 mol-1 = 8.3145 kPa L K-1 mol-1 = 8.3145 J K-1 mol-1
Kw = 1.0 \times 10^{-14} (at 298 K)
F = 96 485 C mol-1
Conversion factors
1 atm = 101.325 kPa = 760 torr = 760 mm Hg
0oC = 273.15 K
Equations
PV = nRT
k t1/2 = 0.693
pH = pKa + log ([base]/[acid])
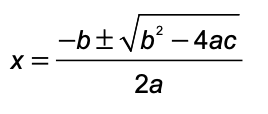
CHEM 13 NEWS EXAM © 2010 UNIVERSITY OF WATERLOO