UNIVERSITY OF WATERLOO
DEPARTMENT OF CHEMISTRY
21 MAY 2009
TIME: 75 MINUTES
This exam is being written by several thousand students. Please be sure that you follow the instructions below.
We'll send your teacher a report on your performance. Top performers are eligible for a prize. The names of the top 200 students will be published in the September issue of Chem 13 News.
- Print your name here: _________________________
- Print your school name and city on your STUDENT RESPONSE sheet.
- Select, and enter on the STUDENT RESPONSE sheet, one of the following CODE numbers:
- Code 1 Ontario, now studying Grade 12 Chemistry in a nonsemestered school
- Code 2 Ontario, now studying Grade 12 Chemistry in a semestered school
- Code 3 Ontario, Grade 12 Chemistry already completed
- Code 4 Any other Ontario student
- Code 5 Manitoba or Saskatchewan high school student
- Code 6 Québec high school student
- Code 7 Québec CEGEP student
- Code 8 Alberta or British Columbia high school student
- Code 9 New Brunswick, Newfoundland, Nova Scotia, or Prince Edward Island high school student
- Code 10 Northwest Territories, Nunavut, or Yukon high school student
- Code 11 High school student outside Canada
- Code 12 Teacher
- Print your name (last name, first name and optional middle initial) on the STUDENT RESPONSE sheet. Also fill in the corresponding circles below your printed name.
- Carefully detach the last page. It is the datasheet.
- Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number.
- Mark only one answer for each question.
- Questions are all of the same value.
- There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer.
- Take care that you make firm, black pencil marks, just filling the oval.
Be careful that any erasures are complete—make the sheet white again.
Carefully detach the last page. It is the Data Sheet.
1. The "lead" of a pencil is mostly
- lead, Pb
- carbon, C
- silicon dioxide, SiO2
- silicon, Si
- calcium carbonate, CaCO3
2. How many protons, neutrons and electrons are there in a single atom of 209Po?
- 84 protons, 84 neutrons, 209 electrons
- 84 protons, 209 neutrons, 84 electrons
- 209 protons, 125 neutrons, 209 electrons
- 125 protons, 84 neutrons, 125 electrons
- 84 protons, 125 neutrons, 84 electrons
3. The mass of one atom of 12C is exactly 12 atomic mass units.
With the assumption that a proton and a neutron are equally massive,
what is the total number of protons and neutrons in the body of a 75-kg person?
(You may neglect the mass of an electron is negligible compared to that of a proton or neutron.)
- 2.2×1027
- 4.5×1028
- 8.0×1021
- 3.8×1023
- 8.0×1024
4. Mercury, Hg(l) has a density of 13.6 g mL-1 at 25°C.
What is volume of 4.25 grams Hg(l) at 25°C?
- 0.0173 mL
- 3.20 mL
- 0.0562 mL
- 0.313 mL
- 0.0735 mL
5. Which of the following molecules has the same number of electrons as a water molecule?
- HF
- BH3
- CO
- H2S
- F2
6. Which of the following elements is a liquid at room temperature and atmospheric pressure?
- chlorine
- phosphorus
- sulfur
- bromine
- iodine
7. What is the formula of the binary compound formed between Mg and P?
- MgP
- Mg2P
- MgP2
- Mg2P3
- Mg3P2
8. Which of the following elements has no known stable compounds?
- neon, Ne
- xenon, Xe
- gold, Au
- platinum, Pt
- uranium, U
9. Which of the following elements is believed to be the most abundant in the earth's crust?
- hydrogen
- oxygen
- carbon
- nitrogen
- silicon
10. Which of the following has the highest concentration at equilibrium when one mole of HCl is dissolved in 1.0 L of water at 25°C?
- Cl−
- Cl+
- Cl2
- H2
- HCl
11. What is the symbol for the atom or ion that results from the addition of two protons to a single atom of 4220Ca?
- 4222Ca2+
- 4422Ca2+
- 4222Ti
- 4422Ti2+
- 4420Ti2+
12. In a mixture of N2 and O2 gases, all the N2 molecules and the O2 molecules have the same
- average speed
- average kinetic energy
- partial pressure
- average molecular mass
- average momentum
13. When ethanol, CH3CH2OH, is burned in excess oxygen, carbon dioxide and water are the only products.
What is the coefficient of O2 when the chemical equation representing the combustion reaction is balanced using the smallest whole number coefficients?
- 1
- 2
- 3
- 7
- none of the above
14. In an experiment, 16 g of methane and 32 g of oxygen react to produce 11 g of carbon dioxide.
A balanced chemical equation for the reaction is given below.
CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g)
What is the percentage yield of carbon dioxide in this experiment?
- 10%
- 25%
- 50%
- 67%
- 75%
15. If an oxide of nitrogen contains 25.9% by mass of nitrogen, what is its empirical formula?
- NO
- N2O
- NO2
- N2O4
- N2O5
16. What is the percentage by mass of sodium in a mixture containing 1.00 mol NaCl and 1.00 mol NaF?
- 39.3%
- 45.8%
- 47.1%
- 50.0%
- 54.8%
17. When the hydrides of the group 16 elements are arranged in order of increasing boiling point, the order is
- H2S H2Se H2Te H2O
- H2O H2S H2Se H2Te
- H2Te H2Se H2S H2O
- H2O H2Te H2Se H2S
- H2S H2O H2Se H2Te
18. How many unpaired electrons are there in a ground state Mn2+ ion?
- zero
- one
- two
- three
- more than three
19. What is the pressure (in mmHg) of the gas inside the apparatus below if Patm = 750 mmHg, Δh1=40 mm and Δh2=30 mm?
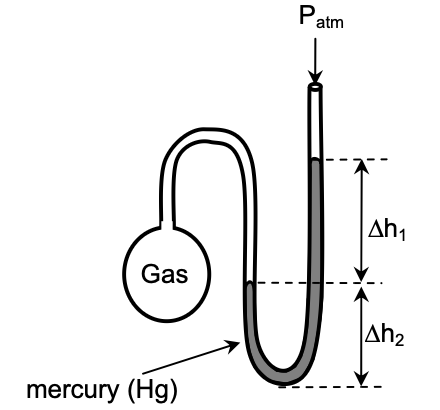
- 710 mmHg
- 790 mmHg
- 720 mmHg
- 780 mmHg
- 820 mmHg
20. What is the HCH bond angle in a formaldehyde (H2CO) molecule?
Choose the closest value.
- 45°
- 90°
- 109°
- 120°
- 180°
21. Which of the following diatomic molecules has the strongest bond?
- N2
- O2
- F2
- Cl2
- Br2
22. Which of the following molecules or ions is planar?
(The central atom is underlined and all other atoms are bonded to it.)
- NH3
- NH4+
- SF4
- SO32-
- SO3
23. What is the formula of iron(II) sulfate?
- Fe2S
- FeS2
- FeSO4
- FeSO3
- Fe2(SO4)3
24. The pH of lemon juice is about 2.3.
What is [H+] in lemon juice?
- 0.36 mol L-1
- 0.83 mol L-1
- 0.10 mol L-1
- 5.0×10-3 mol L-1
- 0.071 mol L-1
25. Solid aluminum dissolves in hydrochloric acid solution according to the following chemical equation.
2 Al(s) + 6 HCl(aq) → 2 AlCl3(aq) + 3 H2(g)
A reaction mixture contains 0.500 mol HCl and 0.400 mol Al.
Assuming the reaction goes to completion, how many moles of the excess reactant remain?
- 0.000 mol
- 0.100 mol
- 0.167 mol
- 0.233 mol
- 0.400 mol
26. What volume does 11 kg of carbon dioxide occupy at 0°C and 101.3 kPa?
- 246 m³
- 5.6×10³ L
- 11 L
- 0.25 L
- 0.22 m³
27. What is the ground state electron configuration of an isolated sulfur (S) atom?
- 1s²2s²2p²3s²3p²4s²3d²4p²
- 1s²2s²2p⁶3s¹3p³3d⁵
- 1s²2s²2p⁶3s²3p⁴
- 1s²2s²2p⁶3s²3p⁶
- 1s²2s²2p⁶3s²3d⁶
28. What volume of 0.123 mol/L aqueous H₂SO₄ is needed to neutralize 40.0 mL of 0.175 mol/L aqueous NaOH?
A balanced chemical equation for the reaction is given below
H₂SO₄(aq) + 2 NaOH(aq) → Na₂SO₄(aq) + 2 H₂O(l)
- 28.5 mL
- 56.9 mL
- 114 mL
- 80.0 mL
- 40.0 mL
29. Three successive elements, in order of increasing atomic number, have these first ionization energies:
1680 2080 494 kJ/mol
Which of following sets represents the three elements?
- N O F
- O F N
- Ne Na Mg
- F Ne Na
- Na Mg Al
30. Which of the following gases does not burn, does not support combustion, and has no effect on lime water, Ca(OH)₂(aq)?
- hydrogen, H₂
- oxygen, O₂
- carbon monoxide, CO
- nitrogen, N₂
- carbon dioxide, CO₂
31. Which of the following elements would you expect to be the most similar in chemical properties to element 20?
- element 19
- element 21
- element 18
- element 4
- element 38
32. A weather balloon filled with helium gas, He(g) has a volume of 2.00×10³ m³ at ground level where the atmospheric pressure is 1.000 atm and the temperature is 27°C.
After the balloon rises high above the earth to a point where the atmospheric pressure is 0.400 atm, its volume increases to 4.00×10³ m³.
What is the temperature of the atmosphere at this altitude?
- -33°C
- -22°C
- -73°C
- 22°C
- 240°C
33. In which of these compounds is the oxidation state of O the highest (i.e., the most positive)?
- F₂O
- O₂
- O₃
- H₂O₂
- H₂SO₄
34. The molar volumes of C2H6(g) and H2(g) measured at 300 K and 10.0 atm, are 2.30 L and 2.51 L, respectively.
Which of the following statements accounts for the observation that the molar volume of C2H6(g) is smaller than that of H2(g)?
- C2H6 molecules are larger than H2 molecules.
- The intermolecular attractions in C2H6(g) are weaker than they are in H2(g).
- The intermolecular attractions in C2H6(g) are stronger than they are in H2(g).
- The average kinetic energy of H2 molecules is greater than that of C2H6 molecules.
- The average kinetic energy of H2 molecules is less than that of C2H6 molecules.
35. When aqueous sodium carbonate, Na2CO3 is treated with dilute hydrochloric acid, HCl, the products are sodium chloride, water and carbon dioxide gas.
What is the net ionic equation for this reaction?
- Na2CO3(aq) + 2 HCl(aq) → 2 NaCl(aq) + CO2(g) + H2O(l)
- CO32-(aq) + 2 HCl(aq) → H2O(l) + CO2(g) + 2 Cl-(aq)
- CO32-(aq) + 2 H+(aq) → H2O(l) + CO2(g)
- Na2CO3(s) + 2 H+(aq) → 2 Na+(aq) + CO2(g) + H2O(l)
- H+(aq) + OH-(aq) → H2O(l)
36. Which of the following is the best Lewis structure (i.e., the best electron dot structure) for the N2O molecule?
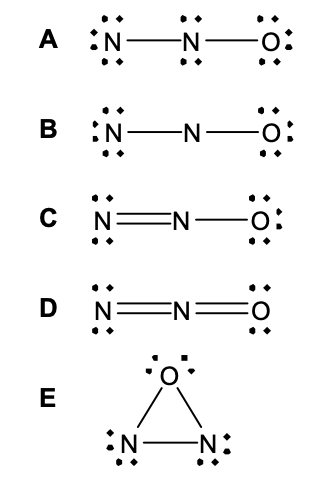
37. A 2.4917-g sample of a hydrate of cobalt (II) fluoride, CoF2⋅xH2O, was heated to drive off all of the water of hydration.
The remaining solid weighed 1.4290 g.
What is the formula of the hydrate?
- CoF2⋅H2O
- CoF2⋅2H2O
- CoF2⋅3H2O
- CoF2⋅4H2O
- CoF2⋅5H2O
38. How many isomers are there for C4H8?
Consider both structural (i.e. constitutional) isomers and stereoisomers.
- one
- two
- three
- four
- more than four
39. Which of the following combinations reagents react to form an insoluble precipitate?
- HNO3(aq) and Ca(OH)2(aq)
- Zn(s) and HCl(aq)
- Zn(s) and Cu(NO3)2(aq)
- NaHCO3(aq) and NaOH(aq)
- Na2CO3(aq) and CaCl2(aq)
40. Which of the following will occur if a 0.10 mol L-1 solution of acetic acid (CH3COOH) is diluted to 0.010 mol L-1 at constant temperature?
- the pH will decrease
- the dissociation constant of CH3COOH will increase
- the dissociation constant of CH3COOH will decrease
- the hydrogen ion concentration will decrease to 0.010 mol L-1
- the percentage ionization of CH3COOH will increase
AVOGADRO EXAM 2009 DATA SHEET
DETACH CAREFULLY
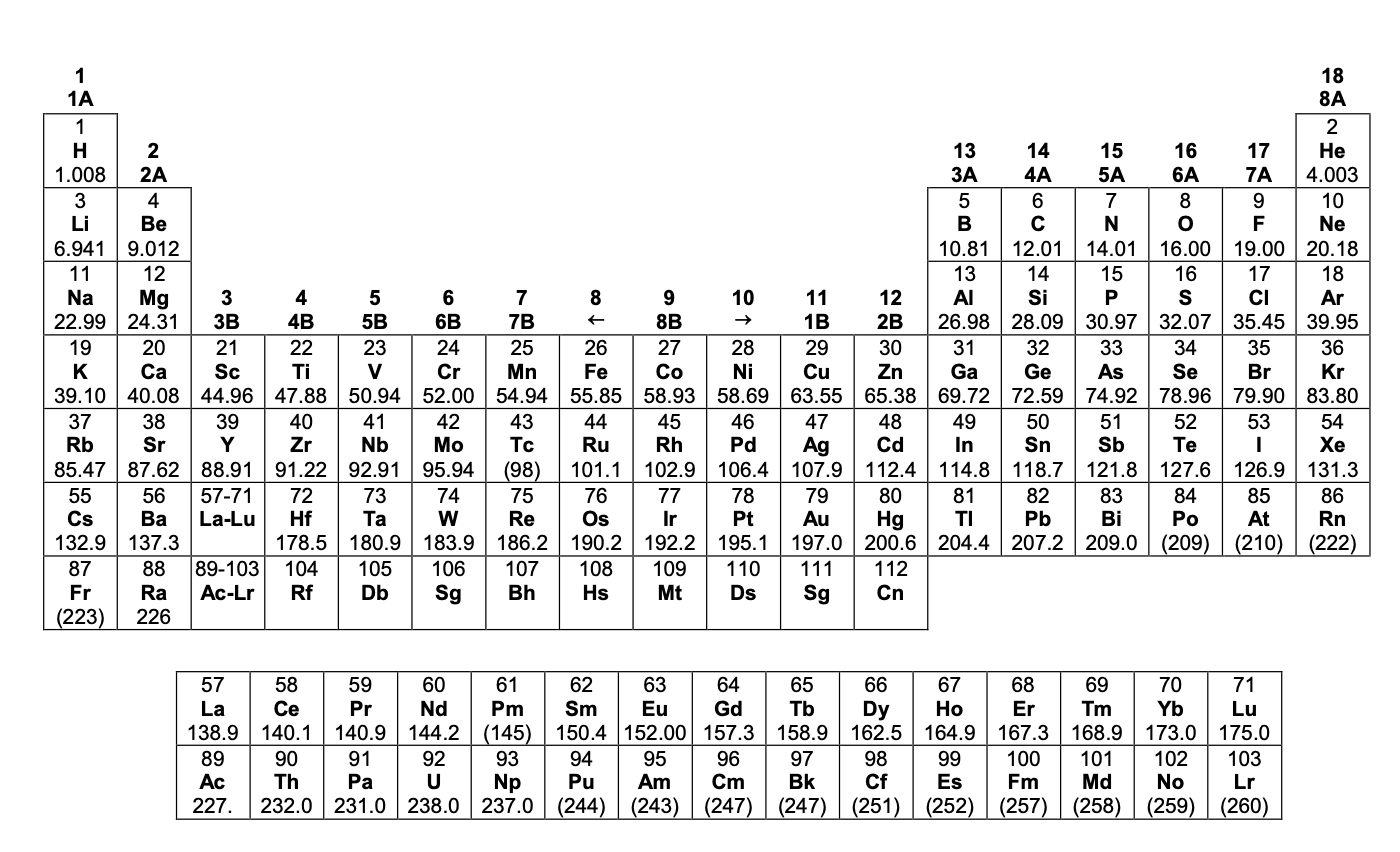
Additional interactive periodic tables
Constants
NA = 6.022 x 1023 mol-1
R = 0.08206 atm L K-1 mol-1 = 8.3145 kPa L K-1 mol-1 = 8.3145 J K-1 mol-1
Kw = 1.0 \times 10^{-14} (at 298 K)
F = 96 485 C mol-1
Conversion factors
1 atm = 101.325 kPa = 760 torr = 760 mm Hg
0oC = 273.15 K
Equations
PV = nRT
k t1/2 = 0.693
pH = pKa + log ([base]/[acid])
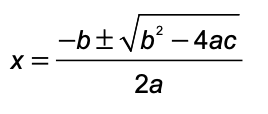
CHEM 13 NEWS EXAM © 2011 UNIVERSITY OF WATERLOO