UNIVERSITY OF WATERLOO
DEPARTMENT OF CHEMISTRY
13 MAY 2010
TIME: 75 MINUTES
This exam is being written by several thousand students. Please be sure that you follow the instructions below.
We'll send your teacher a report on your performance. Top performers are eligible for a prize. The names of the top 200 students will be published in the September issue of Chem 13 News.
- Print your name here: _________________________
- Print your school name and city on your STUDENT RESPONSE sheet.
- Select, and enter on the STUDENT RESPONSE sheet, one of the following CODE numbers:
- Code 1 Ontario, now studying Grade 12 Chemistry in a nonsemestered school
- Code 2 Ontario, now studying Grade 12 Chemistry in a semestered school
- Code 3 Ontario, Grade 12 Chemistry already completed
- Code 4 Any other Ontario student
- Code 5 Manitoba or Saskatchewan high school student
- Code 6 Québec high school student
- Code 7 Québec CEGEP student
- Code 8 Alberta or British Columbia high school student
- Code 9 New Brunswick, Newfoundland, Nova Scotia, or Prince Edward Island high school student
- Code 10 Northwest Territories, Nunavut, or Yukon high school student
- Code 11 High school student outside Canada
- Code 12 Teacher
- Print your name (last name, first name and optional middle initial) on the STUDENT RESPONSE sheet. Also fill in the corresponding circles below your printed name.
- Carefully detach the last page. It is the datasheet.
- Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number.
- Mark only one answer for each question.
- Questions are all of the same value.
- There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer.
- Take care that you make firm, black pencil marks, just filling the oval.
Be careful that any erasures are complete—make the sheet white again.
Carefully detach the last page. It is the Data Sheet.
1. Compared to an electron, a proton has
- the same charge and about the same mass
- the same charge but a much greater mass
- the opposite charge and much less mass
- the opposite charge and a much greater mass
- no charge and a much smaller mass
2. Argon has three isotopes with relative atomic masses of 36.0, 38.0 and 40.0.
Given that the relative atomic mass of naturally occurring argon is 39.95,
which of the following statements must be correct?
- 40Ar is less abundant than 38Ar.
- 40Ar is more abundant than either 36Ar or 38Ar.
- 38Ar is more abundant than 36Ar.
- 36Ar is more abundant than 40Ar.
- Another isotope of lesser mass must exist.
3. An incomplete equation describing the nuclear decay of boron-9 is given below.
How many neutrons or protons are also produced?
95B → 84Be + ?
- one neutron
- one proton
- one neutron and one proton
- two protons
- two neutrons
4. When 50.0 mL of water and 50.0 mL of ethanol are mixed, the total volume is found to be 96.5 mL.
What is the density of this water-ethanol solution?
Densities, in g/mL: Water, 1.00 Ethanol, 0.789
- 1.78 g/mL
- 0.895 g/mL
- 0.211 g/mL
- 3.45 mL
- 0.927 g/mL
5. Which of the following has a linear geometry?
- O3
- NO2-
- C2H2
- H2S
- F2O
6. Which of the following elements has properties that most closely resemble those of calcium, Ca?
- sodium, Na
- potassium, K
- magnesium, Mg
- bromine, Br
- krypton, Kr
7. What is the formula of lead(II) nitrate?
- Pb3N2
- Pb2N3
- Pb2NO3
- Pb(NO3)2
- PbNO3
8. Which of the following reacts with moisture in the air to form acid rain?
- sulfur trioxide, SO3
- nitrogen, N2
- carbon dioxide, CO2
- methane, CH4
- ozone, O3
9. Which of the following is an example of chemical change?
- boiling water
- dissolving alcohol in water
- heating copper metal
- compressing a gas
- rusting of iron
10. What is [Na+] in a solution obtained by mixing 50.0 mL of 0.100 mol/L NaNO3(aq) and 25.0 mL of 0.100 mol/L Na2CO3(aq)?
- 0.133 mol L-1
- 0.200 mol L-1
- 0.300 mol L-1
- 0.167 mol L-1
- 0.125 mol L-1
11. What is the mass of 0.67 mol Na?
- 29 mg
- 15 g
- 10 g
- 23 g
- 0.67 g
12. One litre of oxygen gas is compared to one litre of carbon dioxide gas, both at 25°C and 100 kPa.
Which statement is correct?
- The density of the oxygen gas is greater than that of the carbon dioxide gas.
- On average, the kinetic energy of a carbon dioxide molecule is greater than that of an oxygen molecule.
- On average, a carbon dioxide molecule moves faster than does an oxygen molecule.
- On average, the kinetic energy of carbon dioxide molecule is less than that of an oxygen molecule.
- The two samples contain the same number of molecules.
13. What is the net ionic equation for the reaction of Na2CO3(aq) and CaCl2(aq)?
- Na+(aq) + Cl-(aq) → NaCl(s)
- Na2CO3(aq) + CaCl2(aq) → 2 NaCl(aq) + CaCO3(s)
- Ca+(aq) + CO3-(aq) → CaCO3(s)
- Ca2+(aq) + CO32-(aq) → CaCO3(s)
- 2 Na+(aq) + CO32-(aq) → CO2(g) + Na2O(s)
14. In an experiment, 16.0 g SO2 is treated with 6.0 g O2 and 18.0 g SO3 is obtained.
A balanced chemical equation for the reaction is given below.
2 SO2(g) + O2(g) → 2 SO3(g)
What is the percentage yield of SO3 in this experiment?
- 25%
- 38%
- 67%
- 60%
- 75%
| SO2 | 64.1 |
|---|---|
| O2 | 32.0 |
| SO3 | 96.2 |
Using the molar masses given, the "correct" answer is E. However, the molar mass given for SO3 is wrong. Question 14 was deleted.
15. What amount of C8H10O2N4 contains the same number of C atoms as 2 mol CO2?
- 2 mol
- 8 mol
- 4 mol
- 0.25 mol
- 0.5 mol
16. In which region of the periodic table would you find the elements of highest electronegativity?
- top, left
- top, right
- near the middle
- bottom, left
- bottom, right
17. Which of the following has an odd-number of electrons?
- NO3-
- NO2
- N2O
- NO+
- NO2-
18. What is the correct electron arrangement for a scandium (Sc) atom?
(The first number in each list refers to the number of electrons in the first shell, the second number refers to the number of electrons in the second shell, and so on.)
- 2, 8, 9, 2
- 2, 8, 2, 8, 1
- 2, 8, 8, 3
- 10, 10, 1
- 4, 4, 4, 4, 1
19. A 10.0 L cylinder containing neon gas with a measured pressure of 550 kPa at 298 K is connected through a valve to a 2.50 L cylinder containing 275 kPa of helium gas at 298 K.
The valve is opened and the gases mix with no change in temperature.
What is the final total pressure in the system?
- 277 kPa
- 326 kPa
- 413 kPa
- 495 kPa
- 599 kPa
20. What is the H-N-H angle in the NH3 molecule?
Choose the closest value.
- 45°
- 90°
- 109°
- 120°
- 180°
21. Which of the following molecules has the strongest carbon-carbon bond?
- ethanol, CH3CH2OH
- ethanoic acid, CH3CO2H
- ethane, C2H6
- ethene, C2H4
- ethyne, C2H2
22. Consider the Lewis structure below for the CH3CCH molecule.
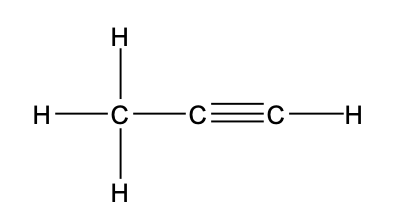
What is the maximum number of atoms that can lie in the same plane?
- three
- four
- five
- six
- seven
23. The following ions all have the same number of electrons. O2-, F-, Na+, Mg2+
In which of following lists are these ions arranged in order of increasing radius (from smallest to largest)?
- O2- < F- < Na+ < Mg2+
- Mg2+ < Na+ < F- < O2-
- Na+ < Mg2+ < O2- < F-
- Mg2+ < Na+ < O2- < F-
- F- < O2- < Na+ < Mg2+
24. Which of the following is not a Bronsted-Lowry conjugate acid-base pair?
- NH3 and NH2-
- OH- and O2-
- H3O+ and OH-
- HCl and Cl-
- NH4+ and NH3
25. A 0.350 g sample of acid HX requires 25.4 mL of 0.140 mol/L NaOH(aq) for complete reaction.
What is the molar mass of the acid?
- 42.3 g/mol
- 68.4 g/mol
- 98.4 g/mol
- 121 g/mol
- 84.6 g/mol
26. What is the density of carbon dioxide gas at 0.00 °C and 101.3 kPa?
- 1.96 g/L
- 0.0446 g/L
- 22.4 g/L
- 44.6 g/L
- 0.509 g/L
27. An element M forms an ion M³⁺.
The atom M and the ion M³⁺ have the same
- number of protons
- number of electrons
- radius
- ionization energy
- chemical properties
28. Methanoic acid, HCOOH, is a weak electrolyte.
In a solution prepared by dissolving 0.10 mol HCOOH in water to make 1.0 L of solution, approximately 4.1% of the HCOOH molecules ionize.
What is the pH of this solution?
- 0.61
- 1.39
- 2.39
- 4.10
- 6.10
29. In March of this year, the International Union of Pure and Applied Chemistry (IUPAC) officially approved the name and atomic symbol (Cn) for element 112.
What is the official name of element 112?
- copernicium
- californium
- cupenium
- cernium
- cuternium
30. Element 114 would be placed directly below lead (element 82).
At the present time, nuclear scientists have managed to synthesize only a few atoms of element 114 at any one time and thus, the physical appearance of a larger sample is not yet known.
Based on its position in the periodic table, element 114 is most likely to be a
- reddish-brown volatile liquid
- a pale yellow green gas
- a colourless crystal
- a gray-silvery metal
- a black powdery solid
31. Sodium hydroxide, NaOH, is most likely found in which household product?
- vinegar
- soap
- bleach
- window cleaner
- drain cleaner
32. A balloon was filled with helium gas to a volume of 3.0 L on a day when the atmospheric pressure was 101 kPa and the temperature was 31°C.
The following day, the atmospheric pressure and temperature were measured as 98.3 kPa and 33°C, respectively.
The volume of the balloon had not changed.
Which of the following statements is consistent with these data?
- Based on the changes in pressure and temperature that occurred, the volume of the balloon would not be expected to change.
- The balloon absorbed some air from the atmosphere.
- Some helium gas leaked out of the balloon.
- Helium atoms in the balloon lost energy to the surroundings.
- Based on the changes in pressure and temperature that occurred, the volume of the balloon should have decreased.
33. To prepare exactly 250 mL of 0.10 mol/L HCl(aq) starting from 1.0 L of 0.20 mol/L HCl(aq), one should
- slowly add exactly 125 mL of 0.20 mol/L HCl(aq) to exactly 125 mL of water.
- slowly add exactly 125 mL of 0.20 mol/L HCl(aq) to about 100 mL of water and then dilute with water to a total volume of 250 mL.
- evaporate 750 mL of water from 1.0 L of 0.20 mol/L HCl(aq).
- slowly add exactly 125 mL of water to exactly 125 mL of 0.20 mol/L HCl(aq).
- add 750 mL of 0.10 mol/L NaOH to 1.0 L of 0.20 mol/L HCl(aq).
For question 33, the intended answer was “B”, but the volume of water was mistakenly given as 200 mL when 100 mL is what was intended. Question 33 was deleted. Answer “A” is not the correct answer because 125 mL of HCl(aq) and 125 mL of water may not give exactly 250 mL of solution because volumes are not exactly additive. See question 4 for an extreme example.
34. Which of the following dilute solutions would allow a chemist to distinguish between dilute solutions of NaCl(aq) and NaNO3(aq)?
- NaOH(aq)
- HCl(aq)
- NH3(aq)
- H2SO4(aq)
- AgNO3(aq)
35. Compared to a chlorine atom, a sodium atom has a larger
- radius
- mass
- number of electrons
- ionization energy
- electronegativity
36. Which of the following bonds has the greatest ionic character?
- C-H
- O-H
- O-F
- H-F
- C-O
37. A compound is found to be 85.62% carbon by mass and 14.38% hydrogen.
What is the simplest formula of this compound?
- CH
- CH2
- CH3
- CH4
- C3H4
38. Mercury(II) sulfide, HgS, is practically insoluble in pure water.
Its solubility at 25°C is probably no more than 3×10-20 g/L.
Of the following quantities of pure water, which is the smallest quantity that could be used to make a saturated solution of HgS?
- 20,000 L
- 1000 L
- 10,000 L
- 2000 L
- 200 L
Molar masses (in g/mol)
-
Hg, 200.6
-
S, 32.07
39. What is the pH of a solution prepared by mixing 50.0 mL of 0.010 mol/L HCl(aq) and 50.0 mL of 0.010 mol/L Ca(OH)2(aq)?
Assume the temperature is 25 °C.
Kw = 1.0×10-14 at 25 °C
- 2.00
- 2.30
- 7.00
- 11.70
- 12.00
40. Consider the Lewis structure below.
What is the charge on this molecule or ion?
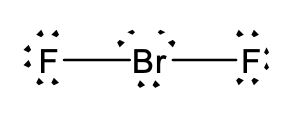
- -2
- -1
- 0
- +1
- +2
CHEM 13 NEWS EXAM 2011 DATA SHEET
DETACH CAREFULLY
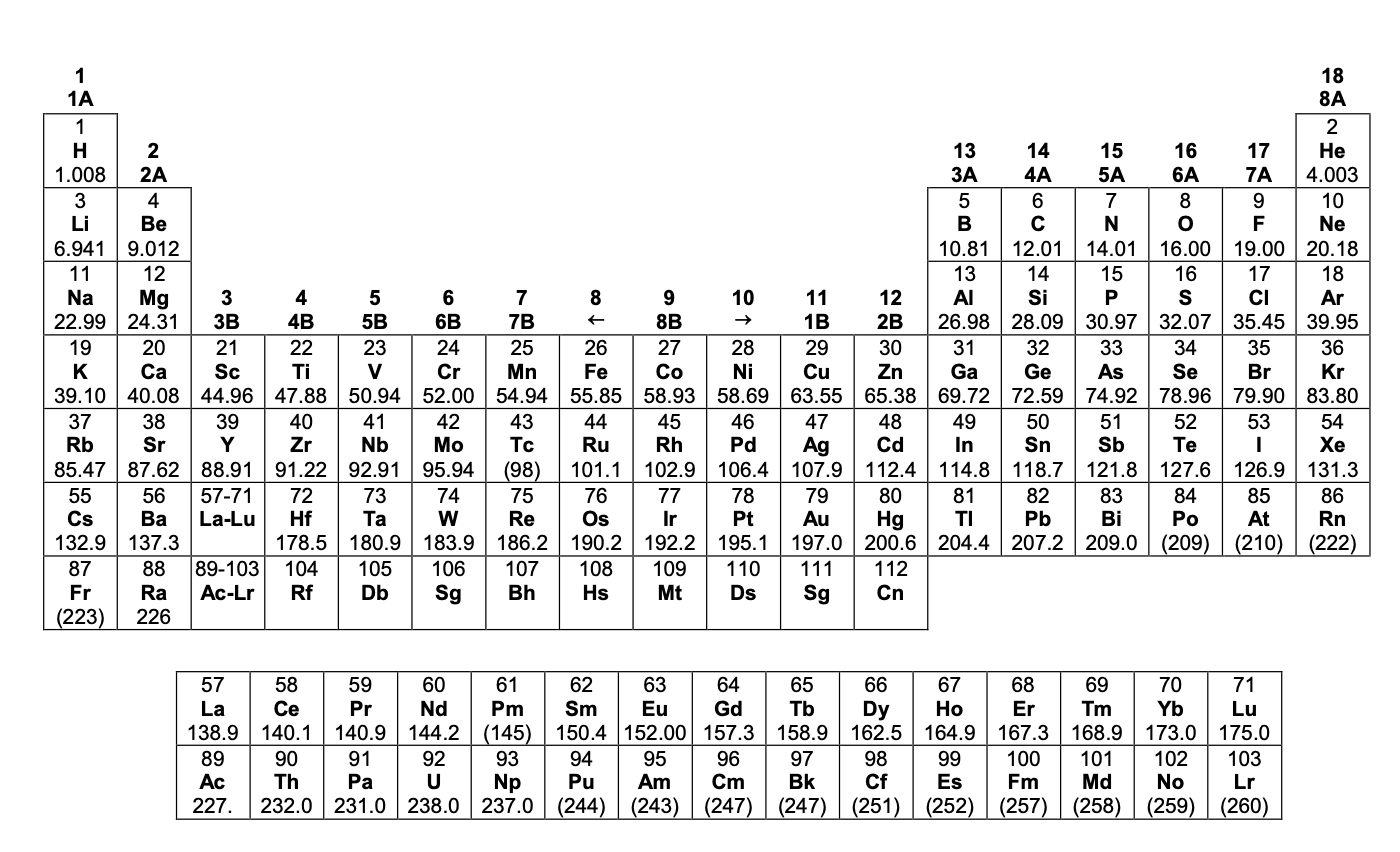
Additional interactive periodic tables
Constants
NA = 6.022 x 1023 mol-1
R = 0.08206 atm L K-1 mol-1 = 8.3145 kPa L K-1 mol-1 = 8.3145 J K-1 mol-1
Kw = 1.0 \times 10^{-14} (at 298 K)
F = 96 485 C mol-1
Conversion factors
1 atm = 101.325 kPa = 760 torr = 760 mm Hg
0oC = 273.15 K
Equations
PV = nRT
k t1/2 = 0.693
pH = pKa + log ([base]/[acid])
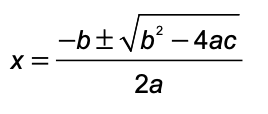
CHEM 13 NEWS EXAM © 2011 UNIVERSITY OF WATERLOO