UNIVERSITY OF WATERLOO
DEPARTMENT OF CHEMISTRY
12 MAY 2011
TIME: 75 MINUTES
This exam is being written by several thousand students. Please be sure that you follow the instructions below.
We'll send your teacher a report on your performance. Top performers are eligible for a prize. The names of the top 200 students will be published in the September issue of Chem 13 News.
- Print your name here: _________________________
- Print your school name and city on your STUDENT RESPONSE sheet.
- Select, and enter on the STUDENT RESPONSE sheet, one of the following CODE numbers:
- Code 1 Ontario, now studying Grade 12 Chemistry in a nonsemestered school
- Code 2 Ontario, now studying Grade 12 Chemistry in a semestered school
- Code 3 Ontario, Grade 12 Chemistry already completed
- Code 4 Any other Ontario student
- Code 5 Manitoba or Saskatchewan high school student
- Code 6 Québec high school student
- Code 7 Québec CEGEP student
- Code 8 Alberta or British Columbia high school student
- Code 9 New Brunswick, Newfoundland, Nova Scotia, or Prince Edward Island high school student
- Code 10 Northwest Territories, Nunavut, or Yukon high school student
- Code 11 High school student outside Canada
- Code 12 Teacher
- Print your name (last name, first name and optional middle initial) on the STUDENT RESPONSE sheet. Also fill in the corresponding circles below your printed name.
- Carefully detach the last page. It is the datasheet.
- Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number.
- Mark only one answer for each question.
- Questions are all of the same value.
- There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer.
- Take care that you make firm, black pencil marks, just filling the oval.
Be careful that any erasures are complete—make the sheet white again.
Carefully detach the last page. It is the Data Sheet.
1. At 25°C and 100 kPa, most of the known elements are
- monatomic gases
- diatomic gases
- liquids
- metallic solids
- non-metallic or semi-metallic solids
2. Which of the following series lists the compounds in order of increasing boiling point? (from lowest to highest)
- H2Te < H2Se < H2S < H2O
- H2S < H2Se < H2Te < H2O
- H2S < H2O < H2Se < H2Te
- H2O < H2S < H2Se < H2Te
- H2O < H2Te < H2Se < H2S
3. In which of the following compounds does oxygen have the highest oxidation state?
- CSO2
- H2O
- O2
- H2O2
- OF2
4. Which of the following processes is the most endothermic?
- H2O(l) → H2O(g)
- F(g) + e- → F-(g)
- NaCl(s) &xrightarrow{H_2O} NaCl(aq)
- Na(g) → Na+(g) + e-
- K+(g) + Cl-(g) → KCl(s)
5. Which of the following atoms has electrons in its outermost shell arranged in the configuration 4s24p3? Assume each atom is in its lowest energy state.
- Rb
- Kr
- As
- Cr
- Sb
6. The following reaction reaches equilibrium in a closed reaction vessel at 200 °C.
CO(g) + 3H2(g) ⇌ CH4(g) + H2O(g), ΔH° = -206 KJ
Which of the following actions causes the reaction to proceed from left to right in order to restore equilibrium?
- increasing the volume of the container, holding temperature constant
- adding some CH4 gas to the system, with volume and temperature held constant
- adding some H2 gas to the system, with volume and temperature held constant
- increasing the temperature, holding the pressure constant
- removing some CO gas from the system, with volume and temperature held constant
7. At a certain temperature, the following equilibrium constants have been measured.
A2(s) + 2 B(g) ⇌ 2 C(g) K1 = 36
D(s) + 2 E(g) ⇌ C(g) K2 = 20
What is the equilibrium constant at the same temperature for the reaction below?
½ A2(s) + B(g) ⇌ D(s) + 2 E(g)
- 720
- 1.8
- 0.56
- 0.30
- 0.090
8. In a particular solution, [Br-] = 0.020 mol L-1 and [CrO42-] = 0.0030 mol L-1.
Finely divided solid silver nitrate, AgNO3, is slowly added to the solution.
What is [Br-] when Ag2CrO4(s) just begins to precipitate?
| Ksp | |
|---|---|
| Ag2CrO4 | 1.9×10-12 |
| AgBr | 5.2×10-13 |
-
2.1×10-8 mol L-1 - 6.0×10-8 mol L-1
- 2.7×10-7 mol L-1
- 5.2×10-13 mol L-1
- 6.4×10-4 mol L-1
9. What is the formula of the stable compound formed by magnesium and nitrogen?
- MgN
- Mg2N
- Mg3N2
- Mg2N3
- MgN2
10. Which of the following ions has the smallest tendency to be protonated when dissolved in liquid acetic acid, CH3COOH(l)?
- hydroxide, OH-
- fluoride, F-
- chloride, Cl-
- bromide, Br-
- iodide, I-
11. X-ray radiation is more energetic than microwave radiation because
- photons of X-ray radiation travel faster than those of microwave radiation
- photons of X-ray radiation are heavier than those of microwave radiation
- X-ray radiation has a higher frequency than does microwave radiation
- X-ray radiation has a longer wavelength than does microwave radiation
- photons of X-ray radiation travel slower than those of microwave radiation
12. Which of the following contains only single bonds?
- NO+
- CO
- CN-
- N22-
- O22-
13. What is the empirical formula of a compound that is 66.64% carbon, 7.45% hydrogen and 25.91% nitrogen by mass?
- C3H4N
- C3H4N2
- C3H3N
- C4H4N
- C4H3N2
14. Let DC=C represent the C=C bond dissociation energy in ethene, H2C=CH2 and DC-C the C-C bond dissociation energy in ethane, H3C-CH3.
How do these bond dissociation energies compare?
- DC=C equals DC-C
- DC=C is exactly equal to 2×DC-C
- DC=C is exactly equal to ½×DC-C
- DC=C is greater than DC-C but less than 2×DC-C
- DC=C is greater than 2×DC-C
15. Which of the following bonds is most polar?
- B-O
- B-F
- C-O
- C=O
- C-F
16. Consider the following energy level diagram for the reaction R → P.
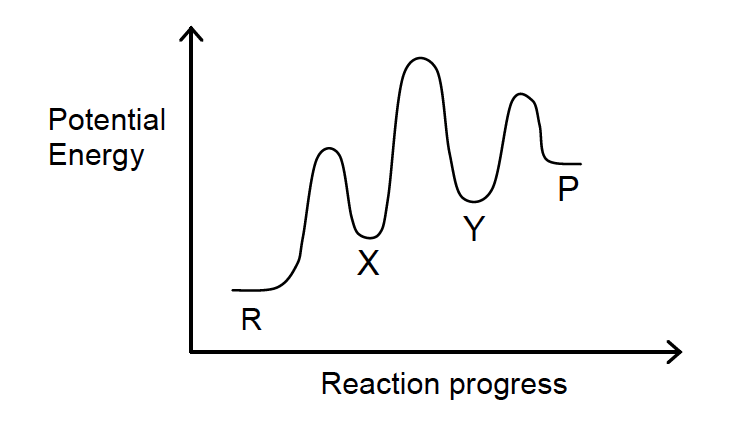
Which of the following statements is false?
- The conversion of R to P occurs via a two-step process.
- X and Y represent reaction intermediates.
- The conversion of R to P is endothermic.
- At equilibrium, the rate of conversion of R to P is equal to the rate of conversion of P to R.
- The rate-limiting step is the conversion of X to Y.
17. A solution in which the bromide concentration is 2.0×10-3 mol L-1 is in equilibrium with solid AgBr and solid AgI.
What is the concentration of iodide ion?
| Ksp | |
|---|---|
| AgBr | 5.2×10-13 |
| AgI | 1.5×10-16 |
- 2.6×10-8 mol L-1
- 5.8×10-9 mol L-1
- 1.5×10-16 mol L-1
- 7.5×10-12 mol L-1
- 2.9×10-4 mol L-1
18. Consider the hydrogen halides HF, HCl, HBr and HI.
Which of the statements about them is true?
- They are all strong acids.
- They are all weak acids.
- The boiling point increases with molar mass.
- The bond dissociation energy increases with molar mass.
- none of above
19. For the reaction below, Kc = 1.0×10-20.
2 A(g) + B(g) ↔ C(g)
In an experiment, 1.0 mol each of A, B and C are placed in an empty 1.0 L container and then the container is quickly sealed.
When equilibrium is established, which of the following will be true?
- [A]<[B]<[C]
- [A]>[B]>[C]
- [A]=[B]=[C]
- [A]=[B]<[C]
- [A]>[B]=[C]
20. What percentage of CH3COOH molecules are ionized in 1.8×10-5 mol L-1 CH3COOH(aq)?
Ka(CH3COOH) = 1.8×10-5
- 1.8%
- 4.2%
- 42%
- 62%
- almost 100%
21. A technician recorded the following curve during a titration.
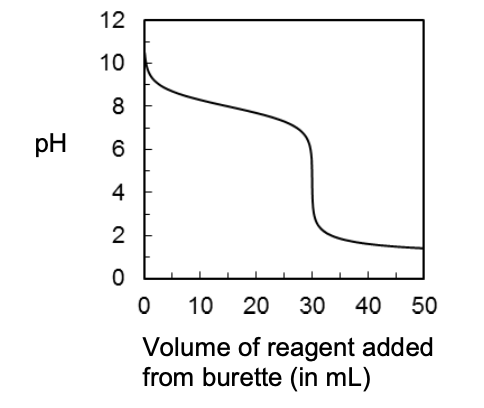
The curve represents the titration of a
- weak acid by adding strong base
- strong acid by adding weak base
- strong base by adding weak acid
- strong base by adding strong acid
- a weak base by adding strong acid
Use the table of standard reduction potentials given below to answer questions 22 through 25.
| Half-Reaction | E° |
|---|---|
| Ag+(aq) + e- ⇌ Ag(s) | +0.80 V |
| O2(g) + 2H2O(l) + 4e- ⇌ 4 OH-(aq) | +0.40 V |
| 2 H+(aq) + 2e- ⇌ H2(g) | 0.0 V |
| Sn2+(aq) + 2e- ⇌ Sn(s) | -0.14 V |
| Ni2+(aq) + 2e- ⇌ Ni(s) | -0.25 V |
| Fe2+(aq) + 2e- ⇌ Fe(s) | -0.41 V |
| Zn2+(aq) + 2e- ⇌ Zn(s) | -0.76 V |
| 2 H2O(l) + 2e- ⇌ H2(g) + 2 OH-(aq) | -0.83 V |
| Al3+(aq) + 3e- ⇌ Al(s) | -1.66 V |
22. Which of the following is the strongest oxidizing agent under standard conditions?
- Ag+(aq)
- Ag(s)
- H+(aq)
- Al(s)
- Al3+(aq)
23. When Ag+(aq) reacts completely with exactly one mole of H2(g) under standard conditions, how many moles of solid Ag are produced?
- 1 mol
- 2 mol
- 0.5 mol
- 4 mol
- 0.25 mol
24. What is E° for the reaction 2 H2(g) + O2(g) ⇌ 2 H2O(l)?
- 1.23 V
- 0.43 V
- 4.06 V
- 0.43 V
- 2.06 V
25. Which of the following reagents would spontaneously reduce Ni2+(aq) to Ni(s) under standard conditions?
- Ag+(aq)
- Ag(s)
- Zn(s)
- Sn(s)
- Al3+(aq)
26. Consider the ions K+, Ca2+, Cl- and S2-. In which series are the species listed in order of decreasing radius? (from largest to smallest)
- S2->Cl->K+>Ca2+
- K+>Ca2+>S2->Cl-
- S2->Ca2+>Cl->K+
- Ca2+>K+>Cl->S2-
- Ca2+>K+>S2->Cl-
27. A solution is prepared by completely dissolving a solid mixture of NaOH and Mg(OH)2 in water.
For the resulting solution, which of the following conditions must be satisfied?
- [Na+] = [Mg2+] = [OH-]
- [Na+] = [Mg2+] = 3 [OH-]
- [Na+] + [Mg2+] = 3 [OH-]
- [Na+] + 2 [Mg2+] = [OH-]
- [Na+] + [Mg2+] = [OH-]
28. What is the minimum volume of water needed to dissolve completely 1.0 g SrF2?
Ksp(SrF2) = 2.8 x 109
Sr, 87.62 g mol-1
F, 19.00 g mol-1
- 9.0 L
- 150 L
- 10.5 L
- 5.6 L
- 2.8 L
29. What is the molecular geometry of SF4?
- T-shaped
- tetrahedral
- see-saw
- square planar
- square pyramidal
30. In the incomplete equation below, NH3 acts as a Bronsted-Lowry acid and "X" represents a Bronsted-Lowry base. What is the conjugate base of NH3?
NH3 + X → ?
- X
- XH+
- NH4+
- NH2-
- OH-
31. What is the general trend observed for the first ionization energies of the elements in groups 13 through 17?
- Ionization energies tend to increase from left to right in a period, and are approximately constant in a group.
- Ionization energies tend to increase from left to right in a period, and decrease from top to bottom in a group.
- Ionization energies tend to decrease from left to right in a period, and increase from top to bottom in a group.
- Ionization energies tend to decrease from left to right in a period, and decrease from top to bottom in a group.
- Ionization energies are approximately constant in a period, and decrease from top to bottom in a group.
32. What is the hybridization of the sulfur atom in the SO32- ion?
- sp
- sp2
- sp3
- sp3d
- sp3d2
33. The phase diagram for an unidentified substance is shown below.
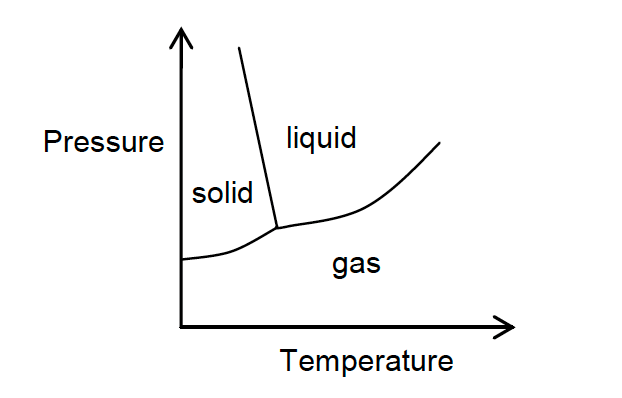
Which of the following statements is true?
- Liquid can be converted to solid by increasing the pressure at constant temperature.
- The melting temperature of the solid increases as pressure increases.
- Solid cannot be converted into gas without first being converted to liquid.
- There is only one combination of temperature and pressure for which solid, liquid and gas can coexist.
- More than one of the statements above are true.
34. When the following equation is balanced using the smallest whole number coefficients, what is the coefficient of O2?
NH3 + O2 → NO + H2O
- 2
- 3
- 4
- 5
- 6
35. What is [CH3COOH] at equilibrium if 0.10 moles of CH3COOH and 0.15 moles of NaOH are dissolved in enough water to make 1.0 L of solution at 25 °C?
For Ka = 1.8×10−5 at 25 °C
- 0 mol L−1
- 1.8×10−5 mol L−1
- 5.6×10−10 mol L−1
- 1.1×10−9 mol L−1
- 1.3×10−3 mol L−1
36. The following diagram is sometimes used to illustrate the structure of benzene, C6H6.
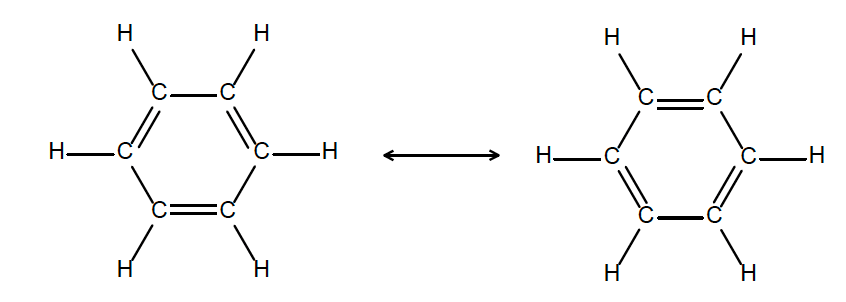
Which of the statements concerning the structure of benzene is false?
- The double bonds oscillate rapidly back and forth between adjacent pairs of carbon atoms.
- The H-C-C angles are 120°
- The carbon atoms form a flat hexagonal ring.
- The oxidation state of carbon is −1.
- The carbon-carbon bonds are all the same length.
37. A particular substance, X, decomposes such that its concentration decreases by a factor of two every 35 s.
If the initial concentration of X was 1.0 mol L−1, what is [X] after exactly 140 s?
- 0.33 mol L−1
- 0.13 mol L−1
- 0.25 mol L−1
- 0.063 mol L−1
- 0.67 mol L−1
38. The bond dissociation energies for F2 and Cl2 are approximately 158 and 242 kJ mol−1 respectively. Given that the enthalpy change for the reaction below is ΔH = −54 kJ mol−1, what is the bond dissociation energy for the F-Cl bond?
½F2(g) + ½Cl2(g) → FCl(g)
- 200 kJ mol−1
- 254 kJ mol−1
- 146 kJ mol−1
- 454 kJ mol−1
- 346 kJ mol−1
39. Which of the following has the greatest number of unpaired electrons in its ground electronic state?
- Al
- Cl
- Ti2+
- Zn2+
- S2−
40. Let HA represent a weak monoprotic acid with Ka = 1.0×10−5.
In an experiment, a 50.0 mL sample of 0.10 mol L−1 HA(aq) is titrated with 0.10 mol L−1 NaOH(aq).
At which point during the titration are the equilibrium concentrations of H+ and OH− equal?
- after the addition of exactly 25.0 mL of NaOH(aq)
- after the addition of slightly less than 50.0 mL of NaOH(aq)
- after the addition of exactly 50.0 mL of NaOH(aq)
- after the addition of more than 50.0 mL of NaOH(aq)
- The equilibrium concentrations of H+ and OH− are never equal.
CHEM 13 NEWS EXAM 2011 DATA SHEET
DETACH CAREFULLY
Additional interactive periodic tables
Constants
NA = 6.022 x 1023 mol-1
R = 0.08206 atm L K-1 mol-1 = 8.3145 kPa L K-1 mol-1 = 8.3145 J K-1 mol-1
Kw = 1.0 \times 10^{-14} (at 298 K)
F = 96 485 C mol-1
Conversion factors
1 atm = 101.325 kPa = 760 torr = 760 mm Hg
0oC = 273.15 K
Equations
PV = nRT
k t1/2 = 0.693
pH = pKa + log ([base]/[acid])
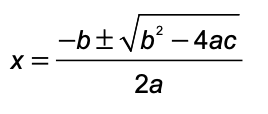
CHEM 13 NEWS EXAM © 2011 UNIVERSITY OF WATERLOO