Background
Metal-containing complexes have been used as catalysts in various organic transformations. These reactions have been demonstrated to be one of the most powerful tools for the synthesis of functional molecules in the fields of biological, pharmaceutical, and material science. While mononuclear complexes (complexes containing a single metal atom) have been studied extensively and applied in various fields and disciplines, bimetallic or multi-metallic systems have received far less attention. Bimetallic complexes are capable of unique organic transformations, high selectively, and can have increased reactivity when compared to analogous monometallic complexes. As bimetallic systems continue to be investigated, the ability to tune key parameters relies on the diversity of binucleating ligands.
Description of the invention
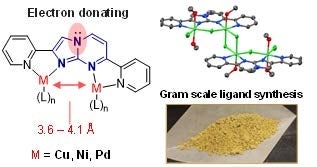
Most existing binucleating ligand designs require synthetic strategies that are complex, low-yielding, and often not environmentally benign. Waterloo researchers have developed a ligand based on the imidazopyrimidine motif that is capable of binding with two (or more) metals with electronically distinct binding pockets. The imidazopyrimidine motif was selected for its convenient synthesis, inherent asymmetry, and increased electron-donating ability. The synthesis of ligand, 2,7-di(pyridin-2-yl)imidazo[1,2-a]pyrimidine (dpip), was completed through the condensation of the corresponding 2-aminopyrimidine and α-bromo ketone, a generalized approach that would allow for convenient modifications without alterations to the synthetic route.
Advantages
The ligand synthesis is scalable, low cost, high yielding and green. It allows for the incorporation of different pendant ligands including pyridines, imines, bipyridine, phosphine etc. The imidazopyrimidine motif can increase the electron-donating ability of the ligand due to resonance contributions from the fused nitrogen, resulting in stronger coordination of the bound metals and increasing electron density at the metal centres.
Catalysts produced with the ligand have inherent asymmetry and different metals (Cu, Ni, Pd, Co, Fe, Rh, Ru etc.) can be incorporated, including those with different sizes and coordination preferences. The ability to incorporate two different metals asymmetrically enables the design of catalysts with unique properties which leads to a broader range of applications.
Potential applications
This technology presents a new class of bi- or multi-metallic ligand capable of forming novel complexes that can offer unique organic transformations, high selectivity, and increased reactivity.
Reference
10244
Patent status
Patent filed
Stage of development
Prototype developed with ongoing research
Contact
Scott Inwood
Director of Commercialization
Waterloo Commercialization Office
sinwood@uwaterloo.ca
uwaterloo.ca/research