The Wettig lab group, led by Professor Shawn Wettig, focuses on design, synthesis, and characterization of compounds called surfactants. Surfactants (short for surface active agents) lower the interfacial tension between two liquids or between a liquid and a solid or air, and are added to products like medications, detergents, skin creams and more. Professor Wettig’s lab specializes in building new types of surfactants with the aim of improving how drugs are delivered into human bodies. Their recent research on surfactants was published as the cover story in the May issue of Physical Chemistry Chemical Physics, a premiere scientific journal put out by the Royal Society of Chemistry.

“Spontaneous organization of molecules lies at the centre of many fundamental aspects of life,” says Samantha Shortall (above), a PhD student in the Wettig Group who co-authored the journal article. “We see this process in the formation of biological membranes and internal structures of living cells, or the formation of proteins like insulin.”
“Physical chemistry and surfactant science allow us to explain such phenomena. We can then mimic these natural systems in a laboratory setting and manipulate the molecules to produce new products for any use we desire - from hair shampoo to furniture polish. Simple changes in the structure of surfactants can have a large impact on their properties such as foaming ability for soaps or temperature solubility in the case of cold water detergents.”
The journal article examines gemini surfactants, a type of surfactant with a unique chemical structure. The structure of gemini surfactants makes them more efficient than traditional surfactants: often, chemists can use less of them to achieve the same effect in a product. For the project, Shortall and the team capitalized on this characteristic of gemini surfactants.
“Over the years, our group has changed various aspects of the surfactants’ molecular structure to improve its efficiency,” explains Shortall. “In this paper, we were able to establish that even counterions, the ions that bind to gemini surfactants, can influence the assembly and physicochemical properties of these molecules.”
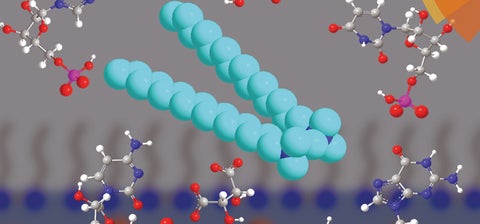
By exploring the different ways that molecular structures can be altered to improve effectiveness of gemini surfactants, the researchers are developing and refining methods that have many applications. Surfactants are relied on by industry for thousands of products, but the Wettig lab is particularly interested in surfactants as a mode of delivering gene therapy.
Right now, gene therapy is typically delivered in one of two ways. One is by viruses – viruses carry desired genes into the body to introduce missing or defective genes. However, viruses sometimes cause immune reactions in patients and are risky for that reason. The second option is delivery by chemical systems, but these methods tend to be less effective than using viruses. Gemini surfactants have the potential to outperform current delivery methods: they form structures that are unlike what current chemical delivery systems contain, and those structures might be more efficient in transplanting the desired genes.
“The work we do is primarily about refining methods and designing compounds that are invisible to the human eye, but can ultimately advance the field of drug delivery and gene therapy,” says Shortall.