Award-winning Waterloo-Hong Kong startup Eyenova Biotech aims to improve early-stage eye drug testing by creating an ‘eye on a chip’

Brandon Ho, Kaya Wong and Liping Zhou (L to R, in black) with Ben Thompson, CEVR's CEO and scientific director (second from left)
The process of getting a drug from the lab to the market is a slow one, often taking a decade or more. An award-winning start-up co-founded by researchers from the University of Waterloo and The Hong Kong Polytechnic University (PolyU) aims to significantly speed up the process when it comes to eye drugs.

Eyenova Biotech, which is supported by the Centre for Eye and Vision Research (CEVR), recently won a gold medal and a China Association of Inventions award at the fourth Asia Exhibition of Innovations and Inventions in Hong Kong. CEVR is a research collaboration between UWaterloo and PolyU funded by the InnoHK Initiative of the Hong Kong Special Administrative Region Government.
The company was founded by Dr. Chau-Minh Phan, a research assistant professor at the UWaterloo School of Optometry and Vision Science and a principal investigator at CEVR; Dr. Kaya Wong, a CEVR-funded postdoctoral fellow at the UWaterloo Department of Chemistry; Dr. Brandon Ho, a scientist at CEVR; and Dr. Liping Zhou, a research assistant professor at PolyU and co-principal investigator at CEVR.
Traditionally, drug testing occurs in three stages. Extensive laboratory cellular testing occurs in the first stage to evaluate the efficacy of candidate drugs. The second phase, animal testing, is costly. Many ophthalmic drugs fail the third phase: human clinical trials.
The company aims to help improve the likelihood of success of candidate drugs going to clinical trials by developing an ‘eye on a chip’ – a preclinical testing platform that replicates the complexity of the eye’s cellular environment and performs multiple tests at once, which saves time, costs and animal testing.
“Our goal is using a combination of microfluidics, cell culture and 3D printing to create a device that mimics the human eye in a miniature form so we can do high-throughput screening of drugs and other new products,” says Ho, the chief technology officer.
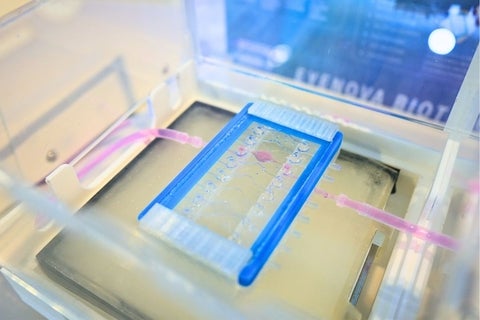
Though lab-on-a-chip platforms have been developed for other organs, the eye has not been well represented in this area to date.
“We aim to speed up drug development by delivering data that’s more clinically relevant than traditional lab or animal testing,” says Wong, Eyenova Biotech’s CEO. “We’re leveraging advanced imaging algorithms to track real-time cellular changes in response to treatment.”
The company’s first-generation device is designed to model dry eye disease, a common and increasingly prevalent condition that can significantly reduce quality of life for patients.
“Our device creates a flow that simulates the movement of tear fluid over the cornea and mimics the actual physiological conditions of the human eye,” says Zhou, Eyenova Biotech’s chief scientific officer.
In 2022, the U.S. Food and Drug Administration introduced a major policy change, eliminating the requirement for animal testing if advanced in vitro models are used. This is part of what spurred the development of Eyenova Biotech.

Incorporated in December 2023, Eyenova Biotech is located within the Hong Kong Science Park.
The founders have big plans to scale up and create even more sophisticated versions of its device.
“It’s difficult to replicate the eye in its entirety, but we can focus on elements that simulate critical factors in different parts of the eye,” says Phan, the chief operating officer, whose PhD research at the School of Optometry and Vision Science laid the foundation for the company. “Our company is creating the systems to facilitate the creation of better products that one day, a lot of people will use.”