Water has many unique properties. An interdisciplinary team of Waterloo scientists has discovered a one-dimensional chain of water molecules could produce a quantum phase transition. This breakthrough is a key development for future water-based quantum devices.
Water has a simple molecular structure - two hydrogen atoms on either side of an oxygen atom - but it also has many unique properties and can exhibit complex behaviours. Many of these behaviours are due to the hydrogen bonds and Van der Waals interaction between the molecules. The angle of the molecule also contributes to its permanent dipole where the hydrogen ends of the molecule are more positive and the oxygen end is more negative.
The molecules dipole strength can be manipulated. Recent studies have shown that water molecules can create interesting quantum phenomenon in lattices when the molecules are far enough apart that the hydrogen bond has no effect but close enough to experience the attraction of an intermolecular dipole interaction.
Waterloo chemists Dr. Pierre-Nicholas Roy and Dr. Tobias Serwatka, a Deutsche Forschungsgemeinschaft (DFG) postdoctoral fellow, collaborated with Dr. Roger Melkoand Dr. Anton Burkov from the Department of Physics and Astronomy. They computed the phase diagram of a linear chain of water molecules after determining the ground and excited orientational states as a function of distance.
Using simulations, the team discovered that a short chain, of only 20 to 50, gaseous water molecules switches from having a random orientation to a highly correlated one.
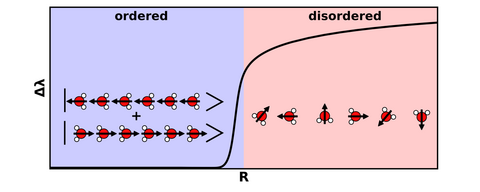
Phase diagram of the one-dimensional water chain where the disordered phase is characterized by molecules delocalized over all orientations at large water-water distances. In contrast, in the ordered phase, the electric dipole moment (black arrows) is aligned leading to a two-fold degenerate ground state which is a superposition of a left- and right- polarized states.
The distance between the water molecules is critical. At large distances, interactions between molecules are very weak and creates a phase of disordered water molecules. However, when the molecules are closer together the molecular dipoles interact more strongly and start to align. Once the molecules reach a critical water-water distance the system crosses the quantum phase transition and reaches a phase of ordered water molecules with aligned parallel dipoles.
The phase transition occurs when the temperature is zero kelvin, eliminating any influence from thermal fluctuations, and the entropy is zero. As a result, the transition is driven only by quantum fluctuations. This quantum phase transition suggests water molecules could be a good candidate for future quantum information processing. The orientation states in a water chain could be used to encode quantum information in a future water-based quantum device. Using water molecules in this way is highly desirable because water is very stable and relatively unreactive.
"The work represents a unique prediction of a quantum phase transition in a molecular assembly associated with the motion of nuclei," says Roy. "The findings will be of interest across boundaries, from atomic and molecular physics, to condensed matters physics and quantum information. A number of experimental realizations of one-dimensional water have already been reported and the work opens the door to the experimental study of quantum criticality and entanglement of molecular rotations."
Their research appeared in Physical Review Letters in January and was selected as an Editors’ Suggestion as well as the focus of a Physics Viewpoint.
The article has been chosen for the Physical Review Journal Club where authors of breakthrough research sit down with the Physical Review Journals and registrants to discuss their research and answer live questions. Postdoc Tobias Serwatka will present these findings on Tuesday March 28th at 2:00 p.m.
Roy is the Canada Research Chair in Quantum Molecular Dynamics and Melko is the Canada Research Chair in Computational Many-Body Physics. Both are affiliate members of the Institute for Quantum Computing. Melko and Burkov are Research Associate Faculty members and Roy is an affiliate member at Perimeter Institute for Theoretical Physics.
This research is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Ontario Ministry of Research and Innovation (MRI) and the Canada Foundation for Innovation (CFI).